Postpneumonectomy syndrome
Introduction
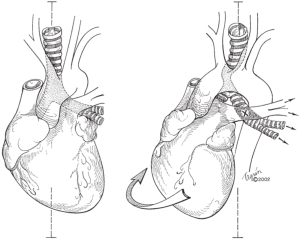
Postpneumonectomy syndrome refers to a constellation of symptoms from airway compression due to mediastinal shifting after pneumonectomy. The heart and mediastinum rotate into the postpneumonectomy space, resulting in two processes: (I) herniation and hyper-expansion of the remaining lung and (II) compression of the airway (trachea, bronchus or lobar orifice) between the pulmonary artery and aorta or vertebral column (Figure 1). Postpneumonectomy syndrome was previously thought a phenomenon limited to patients with left-sided aortic arches who underwent right pneumonectomy (or vice versa), but there have been several case reports of postpneumonectomy syndrome occurring in patients with left-sided aortic arch and left pneumonectomy (1-4). In these cases, the bronchus intermedius was elongated and compressed between the right main pulmonary artery and the thoracic spine.
Prolonged compression and attenuation of the airway can in some cases lead to tracheomalacia or bronchomalacia. In a series of patients from 1979 to 1991 at the Massachusetts General Hospital (MGH) with postpneumonectomy syndrome, 4 out of 11 patients demonstrated significant tracheobronchial malacia; these patients presented 1.4, 8, 17 and 20 years after pneumonectomy compared to the other patients who largely presented within 2 years (5). Interestingly, in this series bronchomalacia was only observed in patients in whom the bronchus was interposed between the pulmonary artery and the aorta (5). In a later series by the same group encompassing 1992 to 2006, none of the 18 patients with postpneumonectomy syndrome demonstrated airway malacia after repair (6).
Clinical presentation
Postpneumonectomy syndrome is a rare late complication of pneumonectomy, with patients presenting up to 20 years after the initial operation (5). The incidence among adults is unknown but in children has been estimated at 0.15% (7) to as high as 17% (8). Classical symptoms of airway obstruction in postpneumonectomy syndrome include dyspnea, stridor, and difficulty clearing secretions, with some patients presenting with episodes of acute respiratory distress. Some patients can present with profound scoliosis. Rarely, patients can present with progressive dysphagia from compression of the esophagus between the heart and aorta (4) or aorta and inferior vena cava (9). There is one report of dysphonia from postpneumonectomy syndrome due to stretch on the contralateral recurrent laryngeal nerve (10).
Unfortunately, the predisposing factors contributing to postpneumonectomy syndrome are unknown. Patients are usually young, though the syndrome has been reported throughout a wide age span, and one hypothesis is that increased mobility of the mediastinum, increased lung compliance and more pliable cartilaginous airway structure in younger patients contribute to airway compression. In one of the largest adult case series containing 18 patients, the median age of presentation was 44 (range 14 to 67) years, the median interval between pneumonectomy and repair was 7.5 (range 1.1 to 54.8) years, the majority of patients were female and most patients had had prior right pneumonectomy (6). Another large series of 20 patients shows similar findings (11) and a summary of case reports up to 2009 showed that 68% of cases occurred after right pneumonectomy (12). The higher incidence in females may could be attributed to the narrower distance between sternum and vertebral body in this population.
Diagnosis
Respiratory insufficiency after pneumonectomy should be investigated thoroughly. The diagnosis of postpneumonectomy syndrome rests upon demonstration of tracheobronchial compression by vascular structures.
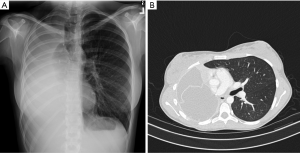
The characteristic findings of postpneumonectomy syndrome on chest X-ray are extreme shifting of the heart and mediastinum into a hemithorax, obliteration of the postpneumonectomy space and overdistension of the remaining lung (Figure 2A). Computed tomography (CT) can be used to identify the point of airway obstruction, for example at the left mainstem bronchus between the pulmonary artery and aorta or against a vertebral body (Figure 2B). Inspiratory and expiratory CT can be helpful to demonstrate dynamic airway obstruction. Shepard et al. codified findings of right postpneumonectomy syndrome on CT as (I) compression of the trachea or of the left main bronchus; (II) rightward mediastinal shift; (III) overdistention of the left lung; (IV) counter-clockwise rotation of the heart and great vessels; and (V) tracheomalacia (13).
Preoperative pulmonary function tests are consistent with airway obstruction with reduction of peak flow, though reduction in forced expiratory volume in 1 second (FEV1) is not consistent (5). Many patients demonstrate increased forced vital capacity (FVC) due to the expansion of the remaining lung, with corresponding decrease in FEV1/FVC ratio (5).
Bronchoscopy is essential to evaluate the postpneumonectomy airway anatomy. Bronchoscopy can show displacement of the trachea, rotation of the carina and should pinpoint the level of airway obstruction. Awake flexible bronchoscopy under topical anesthesia is also valuable in the evaluation of airway malacia to determine the integrity of the cartilages.
Treatment
Once postpneumonectomy syndrome has been diagnosed, treatment should follow promptly to avoid the long-term sequelae of postpneumonectomy syndrome and the acute presentation of respiratory distress. The appropriate current surgical management of postpneumonectomy syndrome requires mediastinal repositioning and filling of the postpneumonectomy space to (I) correct the position of the herniated lung and (II) restore the compressed airway to its normal position and patency. The following points should be emphasized:
- Monitoring. A central venous line and arterial line should be placed to detect hemodynamic changes such as tamponade physiology during mediastinal repositioning, chest closure and changing from lateral decubitus to supine position.
- Complete adhesiolysis of the postpneumonectomy space. Thorough adhesiolysis is required to allow the heart and mediastinum to be repositioned centrally and the remaining lung to fall back to its correct side. One key technical point is that pneumonectomy stump is often tethered to the posterior mediastinum or vertebral body and needs to be fully mobilized for successful airway decompression. Flexible bronchoscopy should be performed to assess airway patency.
- Filling the postpneumonectomy space. Saline-filled breast implants are currently the most widely used prosthetics used to fill the space (4,6,14-17). The volume required is ascertained by instilling a measured amount of saline into the space and is on average 805 (range 225 to 1,610) mL or the equivalent of 2 (range 1 to 4) saline implants (6). Pediatric patients with postpneumonectomy syndrome benefit from insertion of expandable prostheses which allow for progressive adjustments in volume in concert with childhood growth (8,18,19).
- Flexible bronchoscopy should be performed to assess continued airway patency.
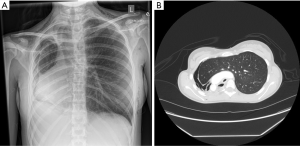
- Final assessment. Once the chest is closed and the patient is in the supine position, bronchoscopy and chest X-ray should be performed to confirm that the mediastinum is midline and that the remaining lung is not atelectatic. Adjustments to the volume of saline implants can be made if needed (Figures 3,4
).
Previously performed techniques to ensure mediastinal repositioning, such as suturing the pericardium to the undersurface of the sternum, abrasion of the endothoracic fascia behind the sternum to promote central fixation and intercostal myoplasty (dropping intercostal muscles into the chest to prevent recurrence) are now deemed unnecessary (5,6). Other methods to ensure midline mediastinum such as crushing the phrenic nerve (20) and aortic suspension (21) have also been unsuccessful. There has been at least one case of successful aortopexy for postpneumonectomy syndrome in our institution where Gore-Tex sling aortoplasty was performed to relieve compression from the aortic arch on the anterior wall of the distal trachea (Cameron D. Wright, personal communication). Aortic division with bypass from the ascending aorta to the descending aorta with a graft to relieve obstruction is associated high mortality (5,6,22). Correction of postpneumonectomy syndrome by endobronchial stenting alone has been demonstrated to have good results at 1 year, but longer-term follow-up is lacking (23-26) and should be used with caution in patients who are otherwise surgical candidates since long-term use of endobronchial stenting for benign conditions usually results in complications such as granulation tissue or migration and can in some cases cause erosion into the aorta or pulmonary artery.
Two recent reports show successful mediastinal positioning and saline prosthesis implantation using thoracoscopic technique (27,28). One patient presented with right postpneumonectomy syndrome 7 months after resection for a congenital abnormality and underwent mediastinal repositioning via two 12 mm thoracoscopic ports but required a 6 cm thoracotomy incision without rib spreading to insert the saline prostheses (28). The second patient presented with right postpneumonectomy syndrome 7 months after resection for carcinoid and underwent mediastinal repositioning via a 12 and a 5 mm port alone; this was achieved by inserting deflated prostheses via the 12 mm port and filling the prostheses with saline intracorporeally (27).
In patients with tracheo- or bronchomalacia after repair, prior series have reported resection of the malacic segment with primary reconstruction (5). The current recommended option for persistent malacia after successful surgical mediastinal repositioning is stenting with completely covered expandable metallic stents, which have demonstrated some success (3,29,30).
Prophylactic placement of saline implants into the fresh pneumonectomy space is not recommended due to the rarity of the syndrome, difficulty in predicting which patients would develop obstructive symptoms and the risk of that prophylaxis may in itself lead to complication, such as infection from foreign body. In the 1940s, methyl-methacrylate spheres were used to prevent overdistension of the remaining lung but caused long-term complications such as tracheal erosion or pseudoaneurysm formation (31). More recently, Memorial Sloan Kettering Cancer Center in New York published a case series of four pediatric patients who underwent prophylactic tissue expander placements immediately after pneumonectomy (32). None of the patients developed postpneumonectomy syndrome. During the short follow-up period (median 13.5 months, range 12 to 52 months), there was no evidence of infection and the only complication was tissue expander rupture at 1 year that did not significantly impact the patient’s clinical course as a capsule had already formed by that time causing midline fixation of the mediastinum (32).
A novel treatment for postpneumonectomy syndrome was reported in 2018 with the use of a customized 3D carbon fiber-printed prosthesis in an 18-year-old female who presented 2 years after a left pneumonectomy for adenoid cystic carcinoma (33). After insertion into the left hemithorax with a Teflon covering, this prosthesis successfully repositioned the mediastinum into a normal position. The authors argue that the improved rigidity and strength of the customized, light-weight (48.6 gram) carbon fiber prosthesis can more reliably maintain stable support of the mediastinum than traditional flexible saline implants (33). Longer-term follow-up is needed to ensure that this rigid prosthesis does not result in erosion of mediastinal structures.
Outcomes
Outcomes after successful mediastinal repositioning and saline prosthesis implantation in patients who do not have residual malacia are good.
Pulmonary function tests before and after repair show consistent increase in peak expiratory flow rate, increased airway diameter and decreased hyperinflation (improved FEV1/FVC ratio and decreased vital capacity) (5,6,12,16). Examining all 23 patients who underwent mediastinal repositioning with placement of prostheses at MGH, 21 out of 23 patients (91.3%) were doing well at up to 12 years of follow-up—one patient required implant removal 1 year after mediastinal repositioning because of dyspnea caused by lobar compression and one patient died of pulmonary embolism 1 month after hospital discharge (5,6). Quality of life questionnaires distributed to the most recent cohort show that 92% of patients reported somewhat better to significant improvement in overall health and breathing postoperatively (6).
In the most recent large series published in 2008, operative mortality was 5.6% (1 patient out of 18 died of multiorgan system failure after aortic division and bypass in a patient with recurrent postpneumonectomy syndrome) and postoperative complications such as pneumonia and acute respiratory failure occurred in 5 out of 18 patients (27.8%) (6). The importance of thorough lysis of adhesions to achieve mediastinal repositioning is demonstrated by a series of 20 patients who underwent repair of postpneumonectomy syndrome in the Netherlands from 1990 to 2005 (11). In this series, 32% of patients had postoperative complications such as recurrence, herniation and inadequate positioning (11).
Conclusions
Patients with symptoms of airway obstruction from postpneumonectomy syndrome should undergo prompt evaluation and treatment. The most effective and durable treatment option is mediastinal repositioning and filling of the postpneumonectomy space. Excellent postoperative outcomes have been reported in patients who do not have residual airway malacia after repair. Novel approaches are required to determine which patients are predisposed to postoperative airway malacia and what may be the best way to manage their persistent airway obstruction.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Lorenzo Spaggiari and Luca Bertolaccini) for the series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.10.07). The series “The Role of Pneumonectomy in Thoracic Surgery in The Third Millennium” was commissioned by the editorial office without any funding or sponsorship. DJM serves as an unpaid editorial board member of Shanghai Chest. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boiselle PM, Shepard JA, McLoud TC, et al. Postpneumonectomy syndrome: another twist. J Thorac Imaging 1997;12:209-11. [Crossref] [PubMed]
- Shamji FM, Deslauriers J, Daniel TM, et al. Postpneumonectomy syndrome with an ipsilateral aortic arch after left pneumonectomy. Ann Thorac Surg 1996;62:1627-31. [Crossref] [PubMed]
- Kelly RF, Hunter DW, Maddaus MA. Postpneumonectomy syndrome after left pneumonectomy. Ann Thorac Surg 2001;71:701-3. [Crossref] [PubMed]
- Bédard EL, Uy K, Keshavjee S. Postpneumonectomy syndrome: a spectrum of clinical presentations. Ann Thorac Surg 2007;83:1185-8. [Crossref] [PubMed]
- Grillo HC, Shepard JA, Mathisen DJ, et al. Postpneumonectomy syndrome: diagnosis, management, and results. Ann Thorac Surg 1992;54:638-50; discussion 650-1. [Crossref] [PubMed]
- Shen KR, Wain JC, Wright CD, et al. Postpneumonectomy syndrome: surgical management and long-term results. J Thorac Cardiovasc Surg 2008;135:1210-6; discussion 1216-9. [Crossref] [PubMed]
- Rasch DK, Grover FL, Schnapf BM, et al. Right pneumonectomy syndrome in infancy treated with an expandable prosthesis. Ann Thorac Surg 1990;50:127-9. [Crossref] [PubMed]
- Podevin G, Larroquet M, Camby C, et al. Postpneumonectomy syndrome in children: advantages and long-term follow-up of expandable prosthesis. J Pediatr Surg 2001;36:1425-7. [Crossref] [PubMed]
- Yüksel M, Yildizeli B, Evman S, et al. Postpneumonectomy esophageal compression: an unusual complication. Eur J Cardiothorac Surg 2005;28:180-1. [Crossref] [PubMed]
- Gullung JL, Halstead LA. Recurrent laryngeal nerve paresis postpneumonectomy contralateral to site of surgery. Ann Thorac Surg 2012;94:628-30. [Crossref] [PubMed]
- Macaré van Maurik AF, Stubenitsky BM, van Swieten HA, et al. Use of tissue expanders in adult postpneumonectomy syndrome. J Thorac Cardiovasc Surg 2007;134:608-12. [Crossref] [PubMed]
- Soll C, Hahnloser D, Frauenfelder T, et al. The postpneumonectomy syndrome: clinical presentation and treatment. Eur J Cardiothorac Surg 2009;35:319-24. [Crossref] [PubMed]
- Shepard JA, Grillo HC, McLoud TC, et al. Right-pneumonectomy syndrome: radiologic findings and CT correlation. Radiology 1986;161:661-4. [Crossref] [PubMed]
- Jansen JP, Brutel de la Rivière A, Alting MP, et al. Postpneumonectomy syndrome in adulthood. Surgical correction using an expandable prosthesis. Chest 1992;101:1167-70. [Crossref] [PubMed]
- Muthialu N, Bulstrode N, Elliott MJ. Intrathoracic saline-filled prosthesis to treat postpneumonectomy syndrome. Asian Cardiovasc Thorac Ann 2015;23:78-81. [Crossref] [PubMed]
- Valji AM, Maziak DE, Shamji FM, et al. Postpneumonectomy syndrome: recognition and management. Chest 1998;114:1766-9. [Crossref] [PubMed]
- Birdi I, Bughai M, Wells FC. Surgical correction of postpneumonectomy stridor by saline breast implantation. Ann Thorac Surg 2001;71:1704-6. [Crossref] [PubMed]
- Lloyd MS, Wallis C, Muthialu N, et al. Treatment of postpneumonectomy syndrome with tissue expanders: the Great Ormond Street Hospital experience. J Plast Reconstr Aesthet Surg 2014;67:725-8. [Crossref] [PubMed]
- Ozcelik C, Onat S, Askar I, et al. Surgical correction of postpneumonectomy syndrome by intrapleural expandable prosthesis in a child. Interact Cardiovasc Thorac Surg 2004;3:390-2. [Crossref] [PubMed]
- Maier HC, Gould WJ. Agenesis of the lung with vascular compression of the tracheobronchial tree. J Pediatr 1953;43:38-42. [Crossref] [PubMed]
- Harrison MR, Hendren WH. A genesis of the lung complicated by vascular compression and bronchomalacia. J Pediatr Surg 1975;10:813-7. [Crossref] [PubMed]
- Horvath P, Dinwiddie R, Stark J. Successful surgical treatment of tracheal compression following right pneumonectomy in infancy. Long-term follow-up. Eur J Cardiothorac Surg 1990;4:351-3; discussion 354. [Crossref] [PubMed]
- Cordova FC, Travaline JM, O'Brien GM, et al. Treatment of left pneumonectomy syndrome with an expandable endobronchial prosthesis. Chest 1996;109:567-70. [Crossref] [PubMed]
- Moser NJ, Woodring JH, Wolf KM, et al. Management of postpneumonectomy syndrome with a bronchoscopically placed endobronchial stent. South Med J 1994;87:1156-9. [Crossref] [PubMed]
- Shah R, Sabanathan S, Mearns AJ, et al. Self-expanding tracheobronchial stents in the management of major airway problems. J Cardiovasc Surg (Torino) 1995;36:343-8. [PubMed]
- Nakamura Y, Ohata M, Kawabe K, et al. Left postpneumonectomy syndrome successfully treated with endobronchial stent. Intern Med 1998;37:880-3. [Crossref] [PubMed]
- Ng T, Ryder BA, Maziak DE, et al. Thoracoscopic approach for the treatment of postpneumonectomy syndrome. Ann Thorac Surg 2009;88:1015-8. [Crossref] [PubMed]
- Reed MF, Lewis JD. Thoracoscopic mediastinal repositioning for postpneumonectomy syndrome. J Thorac Cardiovasc Surg 2007;133:264-5. [Crossref] [PubMed]
- Abe J, Hasumi T, Tanaka R, et al. Long-term outcome of nitinol stenting to treat asphyxia caused by postpneumonectomy syndrome. Respirol Case Rep 2016;5:e00207 [Crossref] [PubMed]
- Harney MS, Lacy PD, O'Neill S, et al. Nitinol stent insertion for post-pneumonectomy syndrome. J Laryngol Otol 2001;115:938-9. [Crossref] [PubMed]
- Johnson J, Kirby CK, et al. The clinical use of a prosthesis to prevent overdistention of the remaining lung following pneumonectomy. J Thorac Surg 1949;18:164-72; Disc., 187-94.
- Choi L, LaQuaglia MP, Cordeiro PG. Prevention of postpneumonectomy syndrome in children with prophylactic tissue expander insertion. J Pediatr Surg 2012;47:1354-7. [Crossref] [PubMed]
- Wang B, Tan S, Yu F. Correction of postpneumonectomy syndrome with tridimensional carbon fiber-printed implant. J Thorac Cardiovasc Surg 2018;155:e135-7. [Crossref] [PubMed]
Cite this article as: Madariaga MLL, Mathisen DJ. Postpneumonectomy syndrome. Shanghai Chest 2020;4:3.