
Recent advances in airway stenting
Introduction
Airway stenting is one of the most commonly performed procedures in interventional pulmonology (IP). An airway stent is a type of endobronchial prostheses used to palliate airway obstruction and maintain airway patency. While airway stent has a long history that can be traced back to the late 19th century, the rapid advancement of stent technology only occurred in the past 25 years.
Implantable airway stents could be dated to late the 1800s, when Trendelenburg and Bond first described implanting an airway prosthesis (1,2). The first clinical application of a T-tube for airway stenting was reported by Montgomery in 1960 (3). In the 1980s, early expandable metallic stents made from stainless steel were introduced (Gianturco Z-stent and Wallstent). However, these stents suffered from several limitations due to unfavorable mechanical properties (4,5). Silicone stent was introduced and popularized by Dumon in 1990s (6). This marked a milestone in airway stent development, as the silicone stent had a favorable biomechanical profile that allowed its wide adoption. However, the placement of the silicone stent requires rigid bronchoscopy, a skillset that is not as universal as flexible bronchoscopy.
Over the past several decades, airway stents have undergone significant development including expansion of indications, improvement of stent manufacturing, and placement/removal techniques. These changes have led airway stenting to become one of the standard therapies in IP. Here, we review the current indications and available airway stents, the challenges with their use, and future developments in airway stents.
Summary of current available airway stents
There are three main classes of airway stents—silicone, bare metallic, and hybrid stents (Figure 1). Metallic stents can be further divided into three generations. The first generation is composed of uncovered balloon-expandable metallic stents made from stainless steel, the second generation consist of partially covered or uncovered self-expandable metallic stents (SEMS) made from nitinol, and the third-generation are the hybrid fully covered SEMS usually made from nitinol with a polymer covering like silicone or polyurethane (PU). The first-generation metallic stents are now rarely used in clinical practice due to the difficulty in removal and associated complications due to the high pressure it exerted on the airway wall. Current commercially available stents on the market are listed in Table 1.

Table 1
| Type of stent (material) | Manufacturer | Pros | Cons | Size (diameter × length) | Placement technique |
|---|---|---|---|---|---|
| Tubular silicone (silicone) | Hood Laboratories, Pembroke, MA, USA | Less granulation | Migration | 9–20 mm × 20–80 mm | RB |
| Boston, Medical Products, Westborough, MA, USA | Less tumor infiltration | Mucus plugging | |||
| Bryan Corporation/Lymol Medical, Woburn, MA, USA | Long-term placement | ||||
| Easily remove | |||||
| Y-stent silicone (silicone) | Boston, Medical Products, Westborough, MA, USA | Less granulation | Migration | Wide range in diameters of tracheal-bronchial-bronchial: 14-10-10 to 18-14-14 | RB; direct laryngoscopy with “pull technique”, modified laryngoscopy and “push technique” |
| Novatech, Grasse, France | Less tumor infiltration | Mucus plugging | |||
| Bryan Corporation/Lymol Medical, Woburn, MA, USA | Long-term placement | ||||
| Easily remove | |||||
| T-tube silicone (silicone) | Boston Medical Products, Westborough, MA, USA | Horizontal limb prevents migration | Granulation | Adult: 10–16 mm vertical limb and 8 or 11 horizontal limb | Tracheostomy and umbilical tape or Kelly technique |
| AERO stent (laser-cut nitinol structure with PU cover) | Alveolus, Inc., Charlotte, NC, USA | Antimigration embed into the mucosa | Migration | 8–20 mm ×15–80 mm | RB |
| Harder to remove | |||||
| Ultraflex stent (nitinol structure partially with/without PU or silicone cover) | Boston Scientific, Natick, MA, USA | Easily placement | Epithelization | 8–20 mm × 20–80 mm | RB/FB |
| Migration | |||||
| Dynamic Y-stent (stainless steel structure with silicone cover), rigid stent | Boston Scientific, Natick, MA, USA | Maintains airway patency well due to rigid profile | Harder to remove | Tracheal limb: 11–15 mm × 110 mm | Requires laryngoscopy during the initial stages of implantation |
| Main bronchi limbs: 8–12 mm × 25–40 (right/left) mm | |||||
| Bonastent stent (nitinol structure with silicone cover), self- expanding | EndoChoice, Alpharetta, GA, USA | Removable | Migration | 10–30 mm × 20–80 mm (fully-covered) | RB/FB, with delivery catheter |
| With ultra-thin delivery catheter | Fracture | ||||
| Resist sever stricture | Mucus plugging | ||||
| Silmet stent (nitinol structure with polyester cover), self-expanding | Novatech, La Ciotat, France | Multiple shapes | Migration | 10–20 mm × 20–60 mm (fully-covered) | RB/FB |
| Fracture | |||||
| Hanaro stent (nitinol structure with silicone cover), self-expanding | M.I.Tech Co., Ltd., Seoul, South Korea | Antimigration flares at both end | Migration | 10–22 mm × 30–80 mm | RB/FB |
| Fracture | |||||
| Micro-Tech stent |
Micro-Tech Co., Ltd., Nanjing, China | Multiple selection | Migration | Sraight | RB/FB |
| 12–18 mm × 40–60 mm | |||||
| Straight | Inexpensive | Fracture | Y-shape | ||
| Y-shape | Easier to deploy | Tracheal limb: 16–20 mm × 40–50 mm | |||
| J-shape | Main bronchi limbs: 12–14 mm × 20/30 (right/left) mm | ||||
| TTS | J-shape | ||||
| Tracheal limb: 16–20 mm × 40–50 mm | |||||
| Main bronchi limbs: 12 mm × 30 mm | |||||
| Balloon-expanding stents (fully covered stainless steel in two layers of polytetrafluoroethylene) | Atrium iCast, Maquet, Getinge, Hudson, NH, USA | For distal airway | Migration | 5–10 mm × 16–38 mm | RB/FB, with the balloon |
RB, rigid bronchoscope; PU, polyurethane; FB, flexible bronchoscope; TTS, through-the-scope.
Interventional pulmonologist should choose the appropriate stent according to the clinical scenario of each patient. Unfortunately, the commercially available stents still suffer from several limitations, which will be discussed in detail. At present, the “ideal stent” does not exist (7). A list of current indications for airway stenting is listed in Table 2.
Table 2
| Benign airway diseases |
| Post-tuberculosis or post-infectious tracheobronchial stenosis |
| PTBS |
| Post-intubation stenosis |
| Post-traumatic stenosis |
| Pseudotumor: papillomatosis, amyloid, hamartomas, bronchialith |
| TBM |
| EDAC |
| Tracheobronchial stenosis: idiopathic, GPA, RP |
| Benign TEF |
| Malignant airway diseases |
| Extrinsic compression by tumor or lymphadenopathy |
| Endobronchial tumor |
| Mixed endobronchial and extrinsic tumor |
| Malignant TEF |
| Relief of post-obstructive pneumonia in patient allowing for inclusion in chemotherapy protocols |
PTBS, post-transplant bronchial stenosis; TBM, tracheobronchomalacia; EDAC, excessive dynamic airway collapse; GPA, granulomatosis with polyangiitis; RP, relapsing polychondritis; TEF, tracheoesophageal fistula.
In China, the first report of airway stent was performed using nitinol spiral metallic stent for malignant tracheal stenosis in 1993 (8). The mainstay of airway stents in China are silicone and SEMS (uncovered and covered). The most common commercially available domestic airway stents are SEMS and hybrid stents from Micro-Tech (Nanjing, China).
Current challenges
Lack of comparative clinical research data
Despite airway stents have been used in clinical practice for several decades, there are many challenges at present. Most importantly, there is a lack of head-to-head clinical trials to compare any given stent to another. Most of the data have only been described in retrospective studies. A large retrospective analysis of incidence of long-term complications among Ultraflex (Boston Scientific, USA), Aero (Merit Endotek, USA), Dumon silicone bronchial and Y-stents (Boston Medical/Novatech, USA) for malignant airway obstruction was performed by Ost et al. from 2005–2010 (9). Aero stents were associated with an increased risk of infection [hazard ratio (HR), 51.98; 95% confidence interval (CI), 1.03–3.81] and silicone tube stents had an increased risk of migration (HR, 53.52; 95% CI, 1.41–8.82). Moreover, silicone stents and lower respiratory tract infections both increased risks of granulation tissue formation. A more recent retrospective cohort study by Lee et al. compared the complications of different types of metallic, silicone (straight, Y-stent, T-tube), and hybrid stents. They found hybrid stents were more likely to migrate [odds ratio (OR), 6.60; 95%, CI: 2.16–20.2] or obstruct by secretions (OR, 2.53; 95%, CI: 1.10–5.84) compared to all other stents (10). Comparative, prospective, randomized controlled trial has yet been performed.
Airway stenting complications
Airway stenting has its complications. There is a wide range (40–60%) of stent-related complication rates in the literature due to differences in study population and the types of airway stents examined (10). The overall rate of complications has been reported to be around 15% (11). This issue is further exacerbated by the significant variability of airway stenting in clinical practice. A large and multicenter cohort study based on the AQuIRE registry examined the incidence of complications of 1,115 procedures with malignant central airway obstruction (CAO) from 15 US centers from 2009–2013, the authors noted that significant practice pattern differences among different centers in airway stent use and associated complications reported (12). This observation is also noted in a European report by Dutau et al. (13).
Most important bronchoscopy-related complications from airway stenting are associated with the technique used during placement and sizing of the stent. Long term stent-related complications (such as mucus plugging, stent migrations or fractures, infections, and obstructions by tumor or granulation tissue) have been reported with metallic, silicone, and hybrid stents. These complications are believed to be due to interruption of mucociliary clearance, over- or under-sizing of stent, stent covering/lack thereof, dynamic nature of airway movement with respiration, and stent biomechanical properties. Yet, no randomized control studies have examined how to minimize these complications. Interestingly, Hoag et al. reported airway stent complications were seen evenly among training groups and practice settings. However, those with increased interventional training are more likely to use chest X-ray to evaluate stent at follow up; hence, more frequently detected stent migration (P=0.036) and mucus plugging (P=0.009) (14). This data suggests that systematic surveillance strategy post-stenting, including stent maintenance and follow-up (see post-stent management), is needed.
Perhaps equally important in minimizing stent-related complications is patient selection. Several factors should be considered to determine whether or when to perform airway stenting. First, whether the airway stenosis is causing clinically significant dyspnea. Tracheal diameter typically has to decrease below 8 mm prior to exertional dyspnea and below 5 mm prior to resting dyspnea/stridor. Second, careful pre-procedural planning is an essential component of airway stenting. Ost et al. reported that urgent and emergent stenting were associated with higher rates of complications than elective procedures (12). Thus, it is best to stabilize and optimize the patient prior to airway stenting. Finally, there should be patent airways distal to the stenosis for stenting from a technical standpoint. In terms of malignant CAO at the end of life, specific goals for the palliative benefit of stents balanced by the complications associated with airway stenting must be addressed with the patient and family (15).
In the near future, improvement of design and material of airway stents could potentially reduce stent-related complications. For example, drug-eluting stent (DES) to reduce granulation tissue formation (16). Recent advances in stent designs will be discussed in detail.
Post-stent management
With increasing use of airway stents, the importance of post-stent management has become more widely recognized. The goal is to facilitate airway clearance (with nebulized saline or hypertonic saline and bronchodilators, with/without acapella valve, with or without guaifenesin). However, there is no standard practice pattern for surveillance and follow-up after stent placement. Long-term follow-up data in post-stent period are limited. Considerable variability exists in both maintenance and surveillance schedules for the post-stent period.
Hoag et al. surveyed 62 members of the American Association of Bronchology and Interventional Pulmonology (AABIP) or attendees at their annual meeting during CHEST 2008. The authors found less than 50% responders have a protocol for post-stent management and significant variability in the practice of post-stent care (14). Seventy-five percent of respondents prescribed inhaled medications for posts-tent maintenance (bronchodilators were most commonly used (59.8%) followed by either humidification (46.8%) or mucolytics (46.8%) (14). Thus far, there are no conclusive studies post-airway stenting to support the use of inhaled steroids or antibiotics to prevent granuloma formation and infection, respectively.
Additionally, the frequency and modality of surveillance after stent placement are highly variable. The surveillance methods consist of physical examination, chest radiography, computed tomography (CT), and bronchoscopy (17). In 2000, Matsuo and Colt found that routine surveillance bronchoscopy within 2–3 months after stent placement did not detect a high incidence of stent-related complications (17). However, recently follow-up data in post-stent (metallic, silicone, and hybrid) period show that surveillance bronchoscopy, regardless of symptomatic status, within 4 to 6 weeks post-stent may be useful for early detection of complications and subsequent management (10). This data included the largest number of follow-up post-stent ever studied. Smaller studies suggested that CT was an accurate, non-invasive method to evaluate airway stents and lumen patency, but it was unable to observe stent epithelialization and granulation formation (18,19). Our practice is to routinely use surveillance bronchoscopy or CT scan at 6 weeks post-stenting. However, the effect of these different surveillance methods on patient’s mortality, morbidity, and overall healthcare costs still remains to be examined.
Stent for complex airway stenosis
Complex airway stenosis is always a challenging clinical scenario. Complex stenosis is defined by the involvement of cartilaginous rings and stenosis length longer than 1 cm (20). Examples are airway stenosis due to inflammatory diseases such as granulomatous polyangiitis [granulomatosis with polyangiitis (GPA) or Wegener’s] or relapsing polyarthritis, multiple distal airway stenosis post-lung transplant, complex tracheobronchial-esophageal fistulas due to infection, stenosis involving carina and/or main bronchus. Diseased airways are deformed anatomically into triangular, slit, or twisted morphology. Perhaps the most challenging aspect of airway stenting for these clinical entities is not the technological placement but the lack of appropriate stents to match the deformed airway. A possible solution could be personalized airway stent for the patient-specific anatomy using three-dimensional (3D) printing technology (discussed below).
Metallic stents in benign airway stenosis
Post-tuberculosis tracheobronchial stenosis and post-traumatic stenosis (after intubation or tracheostomy) are considered to be the most common causes of benign airway stenosis (21). Other less common indications include benign tracheoesophageal fistula (TEF), relapsing polychondritis (RP), excessive dynamic airway collapse (EDAC) or tracheobronchomalacia (TBM), and post-lung transplantation airway stenosis. The causes of benign airway stenosis are listed in Table 2.
Over the past several decades, the application of metallic stent for airway stenosis has fluctuated greatly. The use of metallic stents in benign airway disease was cautioned by United States Food and Drug Association (FDA) in 2005 due to their significant associated complications (22). However, it is noteworthy that at the time of FDA recommendation, the available metallic stents were mostly uncovered and with limited biomechanical properties. With advancements in manufacturing, third-generation metallic stents (hybrid SEMS), such as Bonastent (EndoChoice, USA) and Silmet stent (Novatech, France), were developed. Over the last decade, these hybrid stents have been used to treat benign airway stenosis with satisfactory safety profile.
The recent large series documenting the safety and efficacy of a third-generation SEMS (Silmet stent) in benign airway stenosis was reported by Fortin et al. (23). In their study, 50.0% stents were removed because of stent-related complications after a median of 77.0±96.6 days. About 90.0% stents were removed successfully after a median of 122.0±113.2 days without any complications from the removal procedure. The clinical success rate of stent treatment for benign airway stenosis was 40.7%. A larger, more recent retrospective study from 2003–2016 by Xiong et al., 131 stents were deployed on 116 patients for benign airway stenosis (24). SEMS (Micro-Tech, Nanjing, China) were used in this study. The authors showed acceptable complication profile of SEMS, with most frequently encountered being granulation formation (37.29%). Data at follow-up (median: 1,276 days; range, 2–4,263 days) indicated 68 patients had complete resolution of stenosis, 15 had additional interventional treatment, 8 had bronchial occlusions, 7 underwent surgery, and 4 died of causes unrelated to the stent. These results suggest that the use of hybrid SEMS for benign airway stenosis can be safe and effective long-term.
In general, metallic stents should only be used in benign airway disease when surgery is not possible and silicone stent cannot be used. However, with the evolving third-generation hybrid stent, it is likely that one day soon silicone stent may be replaced. Additional large comparative studies are needed to compare the cost effectiveness and efficacy of new-generation SEMS for benign airway stenosis.
Metallic stents removal
In clinical scenarios where stents need to be removed, the uncovered metallic stents are often very challenging due to epithelialization of the stent into the airway wall. Endoscopic metallic stent removal usually requires rigid bronchoscopy with general anesthesia due to significant complications associated with this procedure (25,26). This has led FDA to issue a black box warning about the use of metallic stent in benign airways (22).
Here are some suggestions with SEMS (covered or uncovered) removal. As a general principle, the stent should be removed in one piece. As such, using fully covered SEMS is referred over the uncovered SEMS, due to the ease with removal process. Second, any ingrown granulation or epithelialized tissue should be removed prior to stent removal (27). The methods treating granulation/epithelialized tissue include thermal modalities (laser, argon plasma coagulation, electrocautery) and cryotechnologies (cryospray/cryotherapy) (25,27,28). Moreover, balloon dilatation could be used to ensure adequate airway and to free the stent from airway wall prior to stent removal (25). Third, one must be cognizant of the different stent removal techniques for the various types of stents. Folch et al. reported covered/partially covered stents (i.e., Aero or Ultraflex) can be removed using twist and pull technique similar to that used in removal of silicone stents (7). Uncovered nitinol stents (Ultraflex) need to be freed from the airway wall with various bronchoscopic techniques and pulling on the proximal circumferential purse-string to collapse proximal end of the stent to facilitate removal.
Perhaps the most important question is when should a stent be removed. This is highly depended on the purpose of stenting and patient factors. Majid et al. recommended short-term (1–2 weeks) use with uncovered nitinol stents with TBM for evaluation benefit to tracheoplasty (29). This is appropriate as epithelization can occur as early as 3–6 week after bare SEMS implantation. Bi et al. suggested a 3-month retention time is reasonable for airway stents placed for post-tracheotomy tracheal stenosis (PTTS) and post-intubation tracheal stenosis (PITS) (30). Ultimately, specific timing of stent removal should be tailored to each patient as stent-related complications may require stent removal earlier than initially intended.
Recent advances
Recent design with airway stents
Fully covered SEMS
The latest fully covered SEMS available in the US is Bonastent (Endo Choice, Alpharetta, GA, USA). Bonastent is composed of nitinol wire weaved using a hook and cross-wire framework fully covered with silicone membrane (Figure 1C). Approved by the FDA in October 2014 and officially launched in the United States in 2016, Bonastent is gaining popularity (31). There are several reported advantages. First, the ability to recapture after up to 70% deployment and conforms to airway without loss of diameter. Second, Bonastent has an ultra-thin delivery catheter [ranging from 2.66 mm (8 Fr) to 5.20 mm (15.6 Fr)] that allows for 10 mm stent to be deployed through the flexible bronchoscope (FB) working channel. Third, multiple different stent sizes (diameter ranging from 10 to 20 mm) fully covered with silicone to reduce tissue in-growth and allowing for easy removal.
To date, only two single-center case series using Bonastent have been published (32,33). Avasarala et al. reported a 13 Bonastents placement in 11 patients. The authors noted 3 incidences of tissue ingrowth through the stent’s covered membrane, 3 incidences of clinically significant mucus plugging, 2 incidences of migration, and 1 incidence of stent fracture (32). Similarly, Makkar et al. reported 13 Bonastents placement in 11 patients. These authors noted 3 cases of mucus plugging, 1 case of stent migration, but no cases of fracture or tumor ingrowth (33). While promising, more data with Bonastent will be needed to fully assess its clinical characteristics.
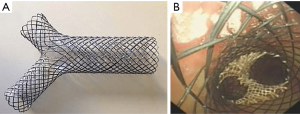
Fully covered metallic Y-stent
A customizable fully covered self-expanding Y-stent (Micro-Tech, Nanjing, China) is available in China and Europe. The stent consists of a nitinol frame with silicone membrane covering (Figure 2). There are limited data available regarding the use of this particular stent. Conforti et al. presented a retrospective case series of 20 consecutive patients with malignant tracheobronchial stenosis who underwent successful placement of fully covered metallic Y-stent under fluoroscopic and/or guidewire guidance (34). Interestingly, Özdemir et al. reported neither fluoroscopic nor guidewire guidance was required for such stent placement via rigid bronchoscopy (35). Several reports in China with larger cohorts demonstrated successful treatment of complex tracheobronchial-esophageal fistulas and airway stenosis with multiple fully covered metallic Y-stent (36,37). Li et al. reported successful treatment of stomach-right mainstem fistulae (close to the right upper lobe take off) with two custom Y-stents (one stent in right mainstem branching into upper lobe and intermediate bronchus, the other in the trachea branching into right and left mainstem) placed under radiographic guidance in 15 patients. The authors noted improvement in patient symptoms, such as cough while lying down and with food ingestion with an average of 26.65 months of follow up (36). Bi et al. showed successful use of full covered Y-stent in 93% (26/28) patients with either gastrobronchial/gastratracheal fistula or bronchopleural fistula or severe tracheobronchial stenosis (30). The authors noted improvement in patient symptoms, no perioperative death, with median survival of 33 months. However, there were 19 complications in 12 patients (43%) that lead to stent removal in 8 patients. Interestingly, complications included stent migration in 2 (7%) and mucus plugging 6 (21%) (37). More experiences with fully covered metallic Y-stents are needed to determine optimal deployment techniques and post-stent management.
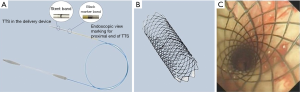
Through-the-scope (TTS) stents
TTS stent is a novel SEMS (Micro-Tech, China) with the delivery system can be directly passed through the flexible bronchoscopy working channel for all available stent sizes. TTS delivery system is achievable only by eliminating guidewire channel to reduce the outer diameter of this delivery system to 2.67 mm. Furthermore, TTS-SEMS ends are in acute angle to aid with loading of the stent into the delivery device (Figure 3). TTS-SEMS uncovered stent goes up to 18 mm in diameter and covered stent goes up to 16 mm in diameter. Jiang et al. reported successful placement of 36 TTS-SEMS (covered and uncovered) in treatment of malignant CAO in 25 patients using a FB with a 2.8 mm working channel under general or local anesthesia (38). Modified Medical Research Council (mMRC) score and stenosis grade improved significantly post-stent placement. Stent-related complications were secretion retention (25.0%, 9/36), development of granulation tissue (13.9%, 5/36), tumor in-growth (13.9%, 5/36, all in uncovered), stent migration (8.3%, 3/36, all in covered), hemoptysis (8.3%, 3/36, all in uncovered). TTS-SEMS delivery can be done under direct visualization resulting in much faster stent deployment, with reported operative time averaging only 60 (range, 25–160) seconds. Additional large cohort studies are needed to further evaluate the stent long term safety profile.
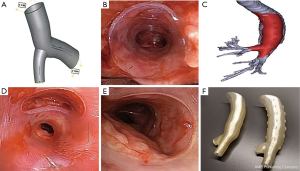
Three-dimensional (3D) printed stents
In the last decade, 3D printing technology or additive manufacturing has been applied in multiple medical specialties. This is also true for airway stenting. The FDA recently released guidelines on the 3D printing of medical devices (39). As mentioned above, complex airway stenosis has been a major challenge in IP. 3D printed stents could address this problem by tailoring the stent to an individual’s airway anatomy creating a personalized stent (Figure 4). Freitag et al. summarized three methods in manufacturing of customized airway stents—direct 3D printing with thermoelastic polymer, 3D printed core silicone dipping, and silicone injection molding (where the mold is 3D printed) (40). There are several recent reports using 3D printed airway prosthesis for stenosis. Cheng et al. successfully designed and placed a 3D designed injection molded Montgomery T-tube for a patient with complex tracheal anastomotic dehiscence (41). This is the first reported case of a personalized T-tube deployed successfully. No granulation tissue or mucus plugging were reported at 1-year follow-up. Additionally, Gildea et al. reported 1-year outcome of 2 patients with silicone stents made from injection molding in 3D printed molds for complex bronchi stricture caused by polyangiitis (Wegener’s) (42). This is the first reported case of an implantable bronchial stent for compassionate use in benign airway disease. Both patients maintained the stents long-term with surveillance bronchoscopy. While exciting, 3D printing is definitely not the last step in the evolution in airway stents. Four-dimensional (4D) printing is being applied to generate printed objects with biological activities for localized pre-programming of tissue differentiation and cell seeding using highly complex smart polymers (40,43). This may be the future for creating biological airway stents and possibly airway replacements.
DESs
DESs were initially developed targeting granulation tissue by inhibiting fibroblast growth (16). Human trials of DES are underway but animal studies have shown some promising results (44). Animal study in canine model with paclitaxel-eluting stent showed decreased granulation tissue formation with paclitaxel-treated stents than that with the regular stents (16). In a separate study, cisplatin-eluting stents were implanted in rabbits. The authors observed a sustained release for >5 weeks as measured by serum levels of cisplatin with only mild mucosal changes in the trachea. This observation suggests potential future application in treatment of malignant airway stenosis (45). In another rabbit model, mitomycin-eluting stent had the least granulation formation and mucus trapping at 12 weeks compared with the control groups (46). However, the efficacy of DES has not been always positive. Sigler et al. reported no difference between sirolimus-eluting stent and bare metal stent in rabbit with regard to quality and quantity of granulation tissue proliferation (47). More recently, Chen et al. successfully designed a DES coating for local release of the antitumor drug gefitinib in vitro (48). A PU sandwich construct was prepared by spray coating method in which gefitinib was embedded between PU support layer. This research suggests that the gefitinib/gefitinib-loaded microspheres incorporated PU constructs are suitable for stent coating, although this study was performed in vitro. Currently, there is no commercially available drug eluting airway stent on market.
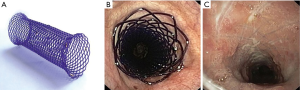
Biodegradable stents (BDSs)
A BDS has been an appealing option as it avoids a separate stent removal procedure. BDS could maintain patency of the airway for a predetermined duration as it gradually degrades to non-toxic byproducts. Currently, BDS are also being studied in drug delivery models. Most BDS has been made with polydioxanone (Figure 5). To date, only a handful of studies have been conducted. Lischke et al. placed 20 BDS (polydioxanone) in 6 adult patients with post lung transplant bronchial stenosis (49). After 4-year follow-up, authors reported a median time to re-stenting of 5 months and median intervention free time of 24 months. All patients were relieved of stenosis initially and no complications peri-stent implantation. Fuehner et al. described a similar series of BDS for 10 adult patients with post-transplant stenosis (50). All patients had symptom relief and improvements in lung function. Stents were completely degraded in a median of 141 days. Sebastian et al. reported a case of BDS with vanishing bronchus intermedius syndrome after lung transplant (51). The authors placed a biodegradable 10×22 mm stent (ELLA-CS, Hradec Kralove, Czech Republic) after Aero stent failure. Airway improved from 20% to 60% patency and stent degraded in 6 months. Given biodegradable and biocompatible characteristics, BDS appears most suitable for the benign airway stenosis, especially in the setting of post-transplant/operative airway stricture and pediatric airway stenosis (52,53). Additional studies will be needed to assess BDS utility in other benign disease conditions and possible roles in malignant disease of adult.
Stent for distal airway stenosis
Airway stenting is well accepted in CAO. Stenting for distal and segmental bronchi have been more controversial. The segmental airways have not been amenable to stenting due to unclear benefits and lack of appropriate stent sizes. However, in recent years, distal airways stenosis associated with lung cancer and post-lung transplantation are increasingly observed. Concurrently, manufacturing improvements have made distal airway stents more readily available. Atrium iCAST stents (Atrium Medical, USA) are balloon-expandable, film-cast encapsulated, fully covered metallic stent (over-balloon) and FDA approved for use in the airway. These stents come in various sizes, from 5 to 10 mm in diameter to 16 to 59 mm in length. In the largest series to date (122 iCAST stents deployed in 38 patients with lobar bronchial stenosis), Sethi et al. showed all patients had symptomatic or radiographic improvement post-stenting. iCAST stent had an acceptable safety profile (10% migration, 5% granulation tissue formation, 2% deployment malfunction, 2% stent dislodgement immediately after deployment, 1% mucous plugging, and 1% tumor occlusion) (54). Additionally, a smaller study by Fruchter et al. demonstrated in 14 patients with SMART nitinol stent (Cordis, USA) and Palmaz stents (Johnson & Johnson, USA) in lobar airways had both significant improvement in their pulmonary function testing and lung functional capacity as measured via 6-min walk test (55). These data suggest stenting for lobar salvage is feasible, safe, and provide clinically significant outcomes. Future larger studies are needed to confirm long-term safety and clinical utility.
Airway stents used in post-transplant airway stenosis
Post-transplant bronchial stenosis (PTBS) is the most common large airway complication after lung transplant with an incidence between 1.6% to 32.0% (56-58). The management of PTBS often requires a stepwise, multimodality approach including dilation, ablation, stent placement, and sometime even repeat transplant. Generally, stent placement is reserved for severe and refractory stenosis. Silicone, fully covered SEMS, and BDS have all been used in treatment of PTBS. While the use of stents has been accepted as an effective therapy for PTBS; however, the timing of removal still remains the question. According to current literature, stent placement with removal after 6 months has been suggested (59).
Bronchial anastomotic dehiscence, another potentially devastating airway complication after lung transplant, has been treated with airway stenting. An uncovered SEMS can be placed temporarily, utilizing SEMS ability to stimulate granulation tissue, to help in healing the defect. Mughal et al. described the successful management of severe dehiscence using Ultraflex uncovered stents. In this study, the authors had observed that these stents can be easily removed by 6–8 weeks after adequate healing has occurred and before epithelialization. The mean time to stent removal was 37.5 (range, 21–54) days (60).
Stent for expiratory central airway collapse (ECAC)
ECAC consists of 2 distinct conditions of the central airways: TBM and EDAC (61). Although the pathophysiology and morphology of TBM and EDAC are different, their clinical symptoms and management are similar. Airway stenting should not be considered as the definitive treatment option for ECAC. While stenting could reduce dyspnea immediately and improve the quality of life, the long-term complications with stenting often worsens the clinical picture and cause more harm. The important value of airway stents for ECAC is to determine the candidacy for tracheobronchoplasty (TBP). Majid et al. demonstrated that use of silicone Y-stent or uncovered Ultraflex stent in patients with severe TBM for a short-term evaluation (approximately 2 weeks) resulted in improved outcomes with few complications and helped in selecting the patient for TBP (29,62). Ozgul et al. reported their experience on silicone Y-stent for severe chronic obstructive pulmonary disease (COPD) complicated with ECAC. Interestingly, changes of forced expiratory volume in 1 second (FEV1) was not significant while mMRC score improvement was statistically significant (P<0.05) (63). ECAC patient should be managed in a systematic fashion, where medical and non-invasive therapy should form the core of treatment, with subgroup of patients undergo rigorous evaluation (including stent trial) prior to definitive surgical correction.
Future of airway stents
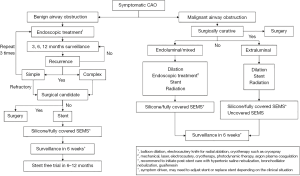
In conclusion, there have been significant advances in airway stents in the past decade. Individualized airway stents for each patient could be available in the future as additional medical-grade materials and manufacturing techniques develops. Truly optimized airway stent with the potential of minimizing long-term complications is within reach. Furthermore, airway stents have become increasingly versatile, particularly in allowing complex and smaller airways to be treated. Despite all the advances, the most important question remains whether the patient requires a stent or not. We propose the following algorithm to determine the need for airway stent in Figure 6. Nonetheless, one should always remember that the best airway stent for a patient is “no airway stent”.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Douglas Kyle Hogarth and Jonathan S. Kurman) for the series “Interventional Pulmonology and Advanced Bronchoscopy” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2019.11.02). The series “Interventional Pulmonology and Advanced Bronchoscopy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Trendelenburg F. Beitrage zu den Operationen an den Luftwegen. Langenbecks Arch Chir 1872;13:335.
- Bond CJ. Note on the treatment of tracheal stenosis by a new T-shaped tracheotomy tube. The Lancet 1891;137:539. [Crossref]
- Montgomery WW. T-tube tracheal stent. Arch Otolaryngol 1965;82:320-1. [Crossref] [PubMed]
- Wright KC, Wallace S, Charnsangavej C, et al. Percutaneous endovascular stents: an experimental evaluation. Radiology 1985;156:69-72. [Crossref] [PubMed]
- Sarodia BD, Dasgupta A, Mehta AC. Management of airway manifestations of relapsing polychondritis: case reports and review of literature. Chest 1999;116:1669-75. [Crossref] [PubMed]
- Dumon JF. A dedicated tracheobronchial stent. Chest 1990;97:328-32. [Crossref] [PubMed]
- Folch E, Keyes C. Airway stents. Ann Cardiothorac Surg 2018;7:273-83. [Crossref] [PubMed]
- Zhang Z, Sun Y, Jiang C, et al. The development of human airway stent and its recent advances. Journal of Changzhou University 2017;29:62-7. (Natural Science Edition).
- Ost DE, Shah AM, Lei X, et al. Respiratory infections increase the risk of granulation tissue formation following airway stenting in patients with malignant airway obstruction. Chest 2012;141:1473-81. [Crossref] [PubMed]
- Lee HJ, Labaki W, Yu DH, et al. Airway stent complications: the role of follow-up bronchoscopy as a surveillance method. J Thorac Dis 2017;9:4651-9. [Crossref] [PubMed]
- Casal RF. Update in airway stents. Curr Opin Pulm Med 2010;16:321-8. [Crossref] [PubMed]
- Ost DE, Ernst A, Grosu HB, et al. Complications following therapeutic bronchoscopy for malignant central airway obstruction: results of the AQuIRE registry. Chest 2015;148:450-71. [Crossref] [PubMed]
- Dutau H, Breen D, Bugalho A, et al. Current practice of airway stenting in the adult population in Europe: a survey of the European Association of Bronchology and Interventional Pulmonology (EABIP). Respiration 2018;95:44-54. [Crossref] [PubMed]
- Hoag JB, Sherman M, Lund ME. Practice patterns for maintaining airway stents deployed for malignant central airway obstruction. J Bronchology Interv Pulmonol 2010;17:131-5. [Crossref] [PubMed]
- Vonk-Noordegraaf A, Postmus PE, Sutedja TG. Tracheobronchial stenting in the terminal care of cancer patients with central airways obstruction. Chest 2001;120:1811-4. [Crossref] [PubMed]
- Wang T, Zhang J, Wang J, et al. Paclitaxel drug-eluting tracheal stent could reduce granulation tissue formation in a canine model. Chin Med J (Engl) 2016;129:2708-13. [Crossref] [PubMed]
- Matsuo T, Colt HG. Evidence against routine scheduling of surveillance bronchoscopy after stent insertion. Chest 2000;118:1455-9. [Crossref] [PubMed]
- Ferretti GR, Knoplioch J, Bricault I, et al. Central airway stenoses: preliminary results of spiral-CT-generated virtual bronchoscopy simulations in 29 patients. Eur Radiol 1997;7:854-9. [Crossref] [PubMed]
- Ferretti GR, Kocier M, Calaque O, et al. Follow-up after stent insertion in the tracheobronchial tree: role of helical computed tomography in comparison with fiberoptic bronchoscopy. Eur Radiol 2003;13:1172-8. [Crossref] [PubMed]
- Dutau H, Musani AI, Plojoux J, et al. The use of self-expandable metallic stents in the airways in the adult population. Expert Rev Respir Med 2014;8:179-90. [Crossref] [PubMed]
- Lim SY, Park HK, Jeon K, et al. Factors predicting outcome following airway stenting for post-tuberculosis tracheobronchial stenosis. Respirology 2011;16:959-64. [Crossref] [PubMed]
- Lund ME, Force S. Airway stenting for patients with benign airway disease and the Food and Drug Administration advisory: a call for restraint. Chest 2007;132:1107-8. [Crossref] [PubMed]
- Fortin M, Lacasse Y, Elharrar X, et al. Safety and efficacy of a fully covered self-expandable metallic stent in benign airway stenosis. Respiration 2017;93:430-5. [Crossref] [PubMed]
- Xiong XF, Xu L, Fan LL, et al. Long-term follow-up of self-expandable metallic stents in benign tracheobronchial stenosis: a retrospective study. BMC Pulm Med 2019;19:33. [Crossref] [PubMed]
- Wang H, Zhou Y, Yamaguchi E, et al. Endoscopic removal of metallic airway stents. J Bronchology Interv Pulmonol 2011;18:31-7. [Crossref] [PubMed]
- Chung FT, Chen GY, Chou CL, et al. Remove airway ultraflex stents by flexible bronchoscope. Am J Med Sci 2012;343:267-72. [Crossref] [PubMed]
- Majid A, Palkar A, Myers R, et al. Cryotechnology for staged removal of self-expandable metallic airway stents. Ann Thorac Surg 2013;96:336-8. [Crossref] [PubMed]
- Noppen M, Stratakos G, D'Haese J, et al. Removal of covered self-expandable metallic airway stents in benign disorders: indications, technique, and outcomes. Chest 2005;127:482-7. [Crossref] [PubMed]
- Majid A, Alape D, Kheir F, et al. Short-term use of uncovered self-expanding metallic airway stents for severe expiratory central airway collapse. Respiration 2016;92:389-96. [Crossref] [PubMed]
- Bi Y, Yu Z, Ren J, et al. Metallic stent insertion and removal for post-tracheotomy and post-intubation tracheal stenosis. Radiol Med 2019;124:191-8. [Crossref] [PubMed]
- Food and Drug Administration. 510(K) summary BONASTENT® tracheal/bronchial. 2014. Available online: www.accessdata.fda.gov/cdrh_docs/pdf14/K140472.pdf
- Avasarala SK, Sethi S, Machuzak M, et al. A single-center case series describing tracheobronchial bonastent implantation. J Bronchology Interv Pulmonol 2019;26:265-72. [Crossref] [PubMed]
- Makkar P, Revelo A, Lee R, et al. A single center experience of feasibility of a novel self-expanding metallic airway stent (bonastent): a case series. J Bronchology Interv Pulmonol 2019;26:254-9. [Crossref] [PubMed]
- Conforti S, Durkovic S, Rinaldo A, et al. Self-expanding Y stent for the treatment of malignant tracheobronchial stenosis. Retrospective study. Arch Bronconeumol 2016;52:e5-7. [PubMed]
- Özdemir C, Sökücü SN, Karasulu L, et al. Placement of self-expandable bifurcated metallic stents without use of fluoroscopic and guidewire guidance to palliate central airway lesions. Multidiscip Respir Med 2016;11:15. [Crossref] [PubMed]
- Li ZM, Lu HB, Ren KW, et al. Thoracic stomach-right main bronchus fistula treated with dual Y-shaped covered airway stents. Clin Radiol 2017;72:517.e1-6. [Crossref] [PubMed]
- Bi Y, Li J, Yu Z, et al. Multiple bifurcated covered self-expanding metallic stents for complex tracheobronchial fistulas or stenosis. Cardiovasc Intervent Radiol 2019;42:426-32. [Crossref] [PubMed]
- Jiang JH, Zeng DX, Wang CG, et al. A pilot study of a novel through-the-scope self-expandable metallic airway stents delivery system in malignant central airway obstruction. Can Respir J 2019;2019:7828526 [Crossref] [PubMed]
- FDA. 3D Printing of Medical Devices. 2018. Available online: https://www.fda.gov/medical-devices/products-and-medical-procedures/3d-printing-medical-devices
- Freitag L, Gördes M, Zarogoulidis P, et al. Towards individualized tracheobronchial stents: technical, practical and legal considerations. Respiration 2017;94:442-56. [Crossref] [PubMed]
- Cheng GZ, Folch E, Brik R, et al. Three-dimensional modeled T-tube design and insertion in a patient with tracheal dehiscence. Chest 2015;148:e106-8. [Crossref] [PubMed]
- Gildea TR, Young BP, Machuzak MS. Application of 3D printing for patient-specific silicone stents: 1-year follow-up on 2 patients. Respiration 2018;96:488-94. [Crossref] [PubMed]
- Devillard CD, Mandon CA, Lambert SA, et al. Bioinspired multi-activities 4D printing objects: a new approach toward complex tissue engineering. Biotechnol J 2018;13:e1800098 [Crossref] [PubMed]
- Colt HG. airway stents. Waltham: UpToDate, 2019. Available online: https://www.uptodate.com/contents/airway-stents
- Chao YK, Liu KS, Wang YC, et al. Biodegradable cisplatin-eluting tracheal stent for malignant airway obstruction: in vivo and in vitro studies. Chest 2013;144:193-9. [Crossref] [PubMed]
- Zhu GH, Ng AH, Venkatraman SS, et al. A novel bioabsorbable drug-eluting tracheal stent. Laryngoscope 2011;121:2234-9. [Crossref] [PubMed]
- Sigler M, Klotzer J, Quentin T, et al. Stent implantation into the tracheo-bronchial system in rabbits: histopathologic sequelae in bare metal vs. drug-eluting stents. Mol Cell Pediatr 2015;2:10. [Crossref] [PubMed]
- Chen W, Clauser J, Thiebes AL, et al. Gefitinib/gefitinib microspheres loaded polyurethane constructs as drug-eluting stent coating. Eur J Pharm Sci 2017;103:94-103. [Crossref] [PubMed]
- Lischke R, Pozniak J, Vondrys D, et al. Novel biodegradable stents in the treatment of bronchial stenosis after lung transplantation. Eur J Cardiothorac Surg 2011;40:619-24. [PubMed]
- Fuehner T, Suhling H, Greer M, et al. Biodegradable stents after lung transplantation. Transpl Int 2013;26:e58-60. [Crossref] [PubMed]
- Fernandez-Bussy S, Labarca G, Majid A. Biodegradable stent for vanishing bronchus syndrome after lung transplantation. J Heart Lung Transplant 2016;35:1378-9. [Crossref] [PubMed]
- Sztanó B, Kiss G, Márai K, et al. Biodegradable airway stents in infants - potential life-threatening pitfalls. Int J Pediatr Otorhinolaryngol 2016;91:86-9. [Crossref] [PubMed]
- Zając A, Krysta M, Kiszka A, et al. Biodegradable airway stents: novel treatment of airway obstruction in children. Adv Clin Exp Med 2019;28:961-5. [Crossref] [PubMed]
- Sethi S, Gildea TR, Almeida FA, et al. Clinical success stenting distal bronchi for "lobar salvage" in bronchial stenosis. J Bronchology Interv Pulmonol 2018;25:9-16. [Crossref] [PubMed]
- Fruchter O, Abed El Raouf B, Rosengarten D, et al. Long-term Outcome of Short Metallic Stents for Lobar Airway Stenosis. J Bronchology Interv Pulmonol 2017;24:211-5. [Crossref] [PubMed]
- Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;6:79-93. [Crossref] [PubMed]
- Machuzak M, Santacruz JF, Gildea T, et al. Airway complications after lung transplantation. Thorac Surg Clin 2015;25:55-75. [Crossref] [PubMed]
- Varela A, Hoyos L, Romero A, et al. Management of bronchial complications after lung transplantation and sequelae. Thorac Surg Clin 2018;28:365-75. [Crossref] [PubMed]
- Fonseca HV, Iuamoto LR, Minamoto H, et al. Stents for bronchial stenosis after lung transplantation: should they be removed? Transplant Proc 2015;47:1029-32. [Crossref] [PubMed]
- Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71. [Crossref] [PubMed]
- Buitrago DH, Wilson JL, Parikh M, et al. Current concepts in severe adult tracheobronchomalacia: evaluation and treatment. J Thorac Dis 2017;9:E57-66. [Crossref] [PubMed]
- Ernst A, Majid A, Feller-Kopman D, et al. Airway stabilization with silicone stents for treating adult tracheobronchomalacia: a prospective observational study. Chest 2007;132:609-16. [Crossref] [PubMed]
- Ozgul MA, Cetinkaya E, Cortuk M, et al. Our experience on silicone Y-stent for severe COPD complicated with expiratory central airway collapse. J Bronchology Interv Pulmonol 2017;24:104-9. [Crossref] [PubMed]
Cite this article as: Liu L, Kong J, George C. Recent advances in airway stenting. Shanghai Chest 2020;4:6.


