
Don’t get your wires crossed: epicardial wire-induced lung granuloma
Introduction
Temporary epicardial pacing wires (TEPWs) are routinely employed in patients undergoing heart surgery, to prevent post-operative dysrhythmias that might result in hemodynamic instability and subsequent cardiac failure. The wires are usually gently pulled out prior to hospital discharge but, in case of difficult removal, they are simply cut at the skin level so that the residual wire retracts into the tissue. Retained TEPWs may migrate into distant organs and cause severe complications. Intrapulmonary migration is rare and has been associated to hemoptysis and recurrent pneumonia.
We present the case of an asymptomatic patient whose chest computer tomography (CT) detected a migrating TEPW and a solid nodule in the right upper pulmonary lobe. The case is presented in accordance with the Case Report (CARE) Guidelines (1).
Case presentation
A 47-year-old woman, former smoker with a history of mitral valve repair, was referred to our Division for a suspicious pulmonary lesion detected on follow-up chest CT. She was asymptomatic and clinical examination was negative. Two months earlier she had undergone mitral valve repair via sternotomy, with TEPWs positioning. CT scans had shown a 7.5-mm spiculated lung nodule in the right upper lobe. Subsequent CT scans at 6 months confirmed the persistence of the lesion and detected a more cranial, infra-centimetric ground-glass opacity (GGO) in the same lung lobe. Surprisingly, further follow-up imaging discovered a cranial migration of the GGO, while both pulmonary lesions stayed stable in size. CT 14 months after the initial retrieval of the lung nodule showed a volume increase of the latter, which proved hypermetabolic on 18F-Fluoro-Deoxy-Glucose Positron Emission Tomography (18F-FDG PET). The GGO, on the contrary, was 18F-FDG PET-negative. Comparison of consecutive multiplanar CT scans reconstructions allowed to identify a foreign body migrating through the right pulmonary parenchyma in caudal-cranial, medial-lateral and anterior-posterior direction and eliciting an inflammatory-like reaction in the surrounding tissues (Figure 1).
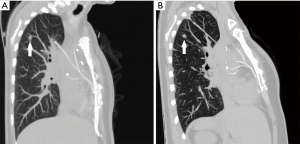
The patient underwent pulmonary atypical resection including both lesions via right thoracotomy. Intraoperative palpation of the upper lobe found caudally the known solid nodule and, cranially, a seemingly inflammatory parenchymal lesion surrounding a foreign body. After cautious extraction, it revealed to be a 20-cm long TEPW (Figure 2). Frozen section disclosed lung adenocarcinoma on the solid nodule, therefore upper lobectomy and systematic hilar-mediastinal lymph node dissection were performed. The post-operative period was uneventful. Following the intraoperative retrieval of the foreign body, amoxicillin/clavulanic acid was initiated. Histopathology confirmed pulmonary adenocarcinoma on the caudal nodule and fibrosis with chronic inflammation on the cranial lesion. Bacterial and mycobacterial cultures on both the TEPW and the parenchymal inflammatory lesion were negative. The patient was discharged 5 days after surgery and fully recovered. Her 10-month post-operative CT findings were consistent with previous lung surgery.
Discussion
Complications related to retained TEPWs are rare (2) and range from cutaneous fistulas to more severe conditions like small bowel obstruction, herniation of intra-abdominal contents into the thorax, intravascular or intrabronchial retention and sepsis (3). Intrapulmonary retention of TEPWs has also been described. Gentry et al. (4) reported the bronchoscopic retrieval and extraction of a TEPW in a man presenting with chest pain and a history of hemoptysis following cardiac operation one year before. Horng and colleagues (5) discovered an endobronchial TEPW while performing flexible bronchoscopy on a man suffering from recurrent pneumonia, who had undergone heart surgery 6 years before. Surgical removal with opening of the pericardium was achieved through thoracotomy, in order to reduce the risk of bleeding related to the long duration of wire retention.
In the presented case, a retained TEPW migrated through the pulmonary parenchyma without crossing any great vessel or mediastinal structure, leaving the patient asymptomatic for 16 months after primary surgery. Due to the peripheric location of both the pulmonary lesions, flexible bronchoscopy was deemed unnecessary. Right thoracotomy was considered the best approach, allowing to diagnose and subsequently treat the pulmonary adenocarcinoma as well as carefully removing the TEPW and preventing complications.
In conclusion, despite its rarity, intrapulmonary wire retention should be considered for the differential diagnosis of GGOs in patients with a history of open-heart surgery. The management of these challenging cases should be carefully planned in a multidisciplinary team, including surgeons, pulmonologists and radiologists.
Moreover, the presence of retained TEPWs should be documented in patients’ medical records and regularly monitored.
Acknowledgments
The authors would like to thank Simona Prisciandaro for proofreading and Andrea Masperi for providing radiological images and reconstructions.
Funding: This work was partially supported by the Italian Ministry of Health with Ricerca Corrente and 5x1000 funds.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc.2020.02.05). LB serves as an unpaid editorial board member of Shanghai Chest from Aug 2019 to Jul 2021. FP serves as an unpaid editorial board member of Shanghai Chest from Jun 2018 to May 2020. LS serves as an unpaid editorial board member of Shanghai Chest from Aug 2019 to Jul 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep. 2013;7:223. [Crossref] [PubMed]
- Omar YA, Wolf LG, Taggart DP. Indications and positioning of temporary pacing wires. Multimedia Man Cardiothorac Surg 2006. doi:
10.1510/mmcts . 2005.001248. - Shaikhrezai K, Khorsandi M, Patronis M, et al. Is it safe to cut pacing wires flush with the skin instead of removing them? Interact Cardiovasc Thorac Surg 2012;15:1047-51. [Crossref] [PubMed]
- Gentry WH, Hassan AA. Complications of retained epicardial pacing wires: an unusual bronchial foreign body. Ann Thorac Surg 1993;56:1391-3. [Crossref] [PubMed]
- Horng GS, Ashley E, Balsam L, et al. Progressive Dyspnea After CABG: Complication of Retained Epicardial Pacing Wires. Ann Thorac Surg 2008;86:1352-4. [Crossref] [PubMed]
Cite this article as: Filippi N, Prisciandaro E, Cavallotti L, Bertolaccini L, Petrella F, Spaggiari L. Don’t get your wires crossed: epicardial wire-induced lung granuloma. Shanghai Chest 2020;4:34.


