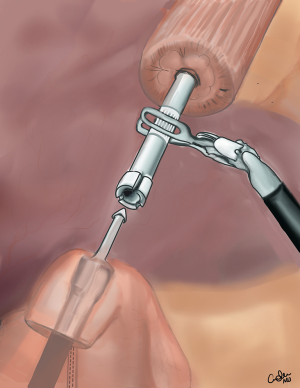
Robotic assisted minimally invasive esophagectomy: Ivor-Lewis approach
Introduction
Esophageal cancer is a highly fatal malignancy that is more common in men and is the 6th leading cause of cancer-related mortality worldwide. In 2019, the Surveillance, Epidemiology and End Results (SEER) Program and American Cancer Society predict 17,650 new cases of esophageal cancer with 16,080 deaths and a reported 5-year survival rate of only 19.9% (1). The geographic distribution, contributing risk factors, and disease presentation can vary significantly based on the histologic subtype, predominantly either squamous cell carcinoma or adenocarcinoma. Although squamous cell carcinoma is more common worldwide, in the Western World including North America, Western Europe, and Australia, adenocarcinoma is the most common histologic subtype correlating with increased rates of obesity and gastroesophageal reflux disease (GERD) (2,3). The strongest risk factor for esophageal adenocarcinoma is obesity, with BMI of 30 having 16 times greater risk compared to a BMI of 22 or less (4). Waist circumference is also associated with proportionally increased risk independent of BMI (4). Additionally, GERD increases the odds of developing adenocarcinoma, with reported risk to be 5 times greater in patients with weekly symptoms, 7 times with daily symptoms, and 9 times in patients with endoscopically confirmed esophagitis (5,6). Barrett’s esophagus was associated with 30 to 60 fold increase in incidence of adenocarcinoma (7).
Esophagectomy for the treatment esophageal cancer was described well over a century ago (8). It continues to be an integral part of the multimodality treatment of esophageal cancer though esophagectomy alone had disappointing survival outcomes, leading to the incorporation of chemotherapy and radiotherapy as part of the multi-modality treatment. The MAGIC and FLOT4 trials have shown increase disease free and overall survival with addition of chemotherapy as neo-adjuvant therapy followed by esophagectomy (9-11). Similarly, the CROSS trial showed significant improvement in long term survival with addition of chemo-radiation as neo-adjuvant therapy (12). Historically, esophagectomy has been associated with high rates of cardiac and pulmonary morbidity and a reported mortality of 8% to 23% in the United States, higher for centers with lower case volume (13). Minimally invasive esophagectomy (MIE) was first described in the 1990s and studies have demonstrated equivalence in safety and outcomes of total laparoscopic/thoracoscopic esophagectomy compared to open technique (14-16). Several studies have demonstrated a decrease in surgical blood loss, chest tube duration, pulmonary complications, and hospital length of stay with MIE when compared to open esophagectomy (OE) (17,18). Luketich et al. compared their minimally invasive McKeown (laparoscopy, VATS, and neck anastomosis) to their modified Ivor Lewis approach (laparoscopy, VATS, and chest anastomosis) with similar outcomes (15).
The last two decades have seen adoption of robotic approach for variety of thoracic procedures including esophagectomy. The robotic approach has the potential advantages of better visualization with three-dimensional optics, increased precision from articulating instruments providing seven degrees of motion, enhanced dexterity, tremor filtration, and telesurgical capabilities that are offered using the DaVinci system (Intuitive, Sunnyvale CA) (19). This is particularly useful for the difficult dissection of the hiatus and mediastinum, where rigid laparoscopic instruments may be restrictive. Robotic assisted minimally invasive esophagectomy (RAMIE) was first described in the early 2000s and there is limited data on the advantages as compared to MIE or traditional open esophagectomy (OE). Several institutions including University of Alabama, Memorial Sloan Kettering, and University of Pittsburgh have established relative safety of the procedure by publishing their single institutional experiences (16,20-24).
Operative technique
RAMIE Ivor-Lewis approach using four-arm robotic techniques with intrathoracic anastomosis using the Orvil 25 mm EEA stapler is the preferred approach for esophagectomy at our institution. The techniques described here utilize the Xi platform (Intuitive, Sunnyvale CA). Here we describe the pre, intra and post-operative details of our approach.
Perioperative planning
All patients with biopsy proven esophageal cancer undergo computed tomography (CT), fluorodeoxyglucose-18 positron emission tomography (PET) and in selected cases, endoscopic ultrasound (EUS). Patients with superficial esophageal cancer (T1a) are considered for endoscopic mucosal resection. If there is submucosal invasion (T1b) then a robotic esophagectomy is offered. Any patient with lymph node involvement or T2 and more advanced lesions are referred for induction treatment. Patients with gastric cardia lesions usually undergo chemotherapy using the FLOT regimen with triplet chemotherapy. The remaining are usually referred for chemotherapy and radiation using Carbo Taxol and 50.4 Gy radiation (modified CROSS trial regime). A repeat CT scan of chest, abdomen and pelvis is performed three weeks after the completion of induction treatment to rule out progression of disease. Patients are placed on full liquid diet for 3 days prior to RAMIE and participate in the institutional ERAS program.
Patients are placed on the operating table in a supine position with both arms out. A foot board is placed to prevent patients from sliding during reverse Trendelenburg position. A double lumen endotracheal tube is used. An endoscopy is performed to evaluate for proximal and distal margins.
Abdominal part of Ivor-Lewis RAMIE
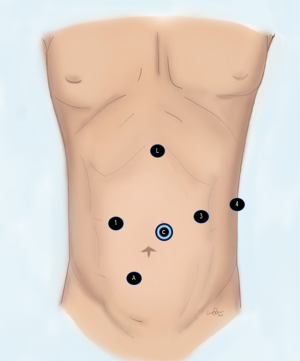
Access to the peritoneal cavity is achieved using a 5 mm access port (Applied Medical, CA, USA) 15 cm from the tip of xyphoid process in mid-clavicular line. This port is later replaced with an 8 mm robotic port and used for the energy device. We use the harmonic scalpel (Johnson & Johnson). Next an 8 mm robotic port is placed 2 cm above and to the left of the umbilicus for the camera. A 12 mm robotic port is placed in the right paramedian region in mid-clavicular line through which a cadiere forceps is used and later robotic stapler is introduced for creation of the gastric conduit. The fourth 8 mm robotic port is placed in the left subcostal area for the double fenestrated tip up grasper. An assistant 12 mm port is created in the right lower quadrant. A Nathanson liver retractor is introduced through a stab wound in the epigastrium and placed under the left lobe of the liver (Figure 1).
The Xi robot is docked. A thorough inspection of the liver and peritoneal cavity is performed to rule out metastasis. The gastro-hepatic ligament is incised and the right crus of the diaphragm is dissected. The phreno-esophageal membrane is incised. The retroesophageal dissection at the level of hiatus is carried out. If possible, authors usually perform circumferential dissection of the esophagus to the level of inferior pulmonary vein to confirm resectability. Next the most proximal short gastric vessels are incised. At this time the dissection is performed at the base of left gastric artery to include all the lymph nodes around celiac axis. Subsequently, the left gastric artery is transected using a vascular Xi robotic stapler introduced through the right paramedian 12 mm port. Dissection is continued toward the left almost skeletonizing the splenic artery to include all the lymphatic tissue in the specimen. The fourth arm is invaluable during this part of the operation and is used to elevate the gastroesophageal junction enabling the surgeon to have an excellent view of retrogastric area all the way to the spleen. At this point the greater curvature is retracted toward the hiatus exposing the gastro-colic ligament. The right gastroepiploic artery is identified and the gastrocolic ligament is incised parallel to that toward the previously incised short gastric. A tongue of omentum along the greater curvature is taken to wrap the anastomosis in the chest. At this point the dissection is carried toward the duodenum, cutting the gastrocolic ligament parallel to the right gastroepiploic artery. Then the avascular plane between the lesser curvature and pancreas is incised which completes the mobilization of the stomach. The authors do not perform a routine pyloric drainage procedure.
The next step is to create the gastric conduit. We use the robotic Xi Stapler. The fourth arm of the robot is used to retract the fundus along the greater curvature. The assistant retracts the greater curvature at the level of the antrum. The first load is always a vascular load transecting the fatty tissue with the right gastric artery up to the lesser curvature. This stapler is placed just left of the first 2–3 branches of the right gastric artery. Next, we use a green load to start fashioning the conduit, aiming the stapler toward the greater curvature and then parallel to it. Subsequently green and blue loads are used to complete the creation of the conduit. We recommend a 4–5 cm gastric conduit. Attention should be paid to prevent spiraling of the conduit as the staplers are fired toward the fundus. The conduit is sutured to the specimen by suturing the tip of the greater curvature of the conduit to the edge of the lesser curvature of the specimen to prevent torsion during the process of pulling the conduit in the chest.
A standard laparoscopic feeding jejunostomy is then performed. The instruments and cords are kept sterile and used during the thoracic part of the procedure.
Thoracic part of Ivor Lewis RAMIE
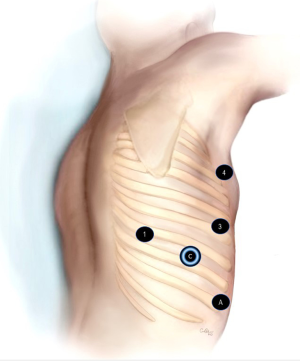
The patient is placed in the left lateral decubitus position, tilted toward the front. A 5mm access port (Applied Medical, CA, USA) is placed in the 8th intercostal space in the posterior axillary line. CO2 insufflation is started at a pressure of 8–10 mmHg. The port is upsized to a robotic 8 mm port for the camera. A 12 mm robotic port is placed in the 6th intercostal space in between mid and anterior axillary line. An 8 mm robotic port is placed in the 3rd intercostal space in the anterior axillary line for the lung retraction. The fourth robotic port is placed in the line of the inferior angle of scapula in the 7th intercostal space. The assistant 12 mm port is placed just above the diaphragm under direct vision between the camera port and the anterior port (Figure 2).
The Xi robot is docked. The dissection is started by incising the inferior pulmonary ligament. The pleura over the posterior surface of the lung is opened and the dissection is continued over the pericardium toward the azygous vein. The subcarinal lymph node pocket is included in the specimen. The use of the harmonic scalpel in the peribronchial area increases the risk of airway injury and future fistula formation. It may be better to either use a bipolar dissector or hook cautery, especially during the initial experience. The azygous vein is transected using a white load introduced through the 15 mm anterior robotic port. Dissection is continued on the posterior side of esophagus. The lymphatics and aorto-esophageal vessels are either cut using the harmonic scalpel or clipped. The dissection is carried down all the way to the hiatus and esophagus is completely mobilized. At this point the gastric conduit is pulled up in the chest using the cadiere forceps and the tip-up double fenestrated grasper on arm 4. Attention is paid to ensure the staple line of the conduit faces the camera to prevent twisting of the conduit. The stitch between the conduit and the specimen is cut and conduit is placed over the diaphragm and secure with a stitch over the diaphragm to prevent it from falling back into the abdomen. Remaining adhesion of the esophagus are lysed and dissection is performed above the level of esophagus. At this point a blue stapler load is introduced and the esophagus is transected just above the azygous vein. The anvil of the 25 mm Orvil (Medtronic, MN USA) mounted on an orogastric tube is passed through the patient’s mouth. A small hole is created in the esophageal stump below the staple line using the hook cautery and the tube is pulled till the metal part of the anvil comes out of that hole. The thread holding the tube and the anvil is cut and the tube is discarded. A purse-string suture is placed around the exit site of the anvil tip.
Next the posterior port site is extended and a small wound protractor is placed. The specimen is removed and sent for frozen section. The tip of gastric conduit is opened and the contents are sucked out. The handle of the 25 mm EEA stapler is passed though the intercostal space and introduced into the gastric conduit through the opening. The handle is introduced deep into the conduit while pushing against the greater curvature to the desired point where the anastomosis is going to be fashioned. At this point the pin of the EEA stapler is brought out along the greater curvature and engaged with the anvil. The stapler is closed and the anastomosis is fashioned (Figure 3). The donuts are checked for completeness and submitted as final proximal margin. A nasogastric tube is placed. The redundant gastric conduit is cut using blue loads 2 cm away from the anastomosis. The fat pad is put between the anastomosis and the trachea. A stitch is placed to narrow the hiatus to prevent future para conduit hernias. The pleural space is irrigated with antibiotic solution. The intercostal are injected with local anesthetic. A Jackson-Pratt (JP) drain is placed between the esophagus and the spine. A chest tube is placed.
Post-operative course
Patients are extubated in the operating room. They are monitored in the intensive care unit for 24 hours. The tube feeds are started on the first post-operative day. The nasogastric tube is removed and then Barium swallow performed on post-operative day 4 or 5. The chest tube is removed after the swallow and patients are started on a limited liquid diet. They are discharged the next day on nocturnal tube feeds and an advancing diet. The patients are seen a week later and the JP drain is removed. The jejunostomy tube is removed 4 weeks after surgery.
Discussion
The first reported RAMIE was in 2002 by Melvin and colleagues, with intra-thoracic anastomosis (25). In 2007, Kernstine et al. reported a series of 14 patients who underwent completely robotic esophagectomy with three-field lymphadenectomy. The total operating time in this group was reported to be 660 minutes, which was markedly increased compared to laparoscopic technique. However, all patients underwent R0 resection with an average of 18 lymph nodes, indicating the robotic approach may facilitate an extended lymphadenectomy (22). In contrast, Weksler and colleagues published their experience with RAMIE compared to MIE for esophageal cancer (26). This study found no significant differences in operative time, number of resected lymph nodes, postoperative complications, or length of stay between the two groups.
Different robotic techniques have been described. Dunn et al. adopted the transhiatal esophagectomy approach. Their outcomes from 40 patients in 2013 included an average operative time of 313 minutes and hospital stay of 9 days. R0 resection was achieved in 94.7% of patients and mean number of lymph nodes dissected was 18, as found in previous studies (27). The technique described by Puntambekar and colleagues in 2015 used the robotic platform for transthoracic mobilization of the thoracic esophagus and lymphadenectomy, with an open cervical anastomosis. In 83 patients who underwent this operation, mean operative time was 205 mins, mean length of stay 10 days, and mean lymph node yield of 18 (28).
Robotic assisted Ivor Lewis (RAIL) combines VATS, laparoscopy, and an intrathoracic anastomosis and is the most frequently reported technique. In the de la Fuente series, 50 patients underwent a robotic assisted Ivor Lewis (RAIL) esophagogastrostomy with a stapled intrathoracic anastomosis. Outcomes included a mean operative time of 445 minutes, length of stay of 9 days, 20 lymph nodes harvested, and 100% R0 resection (29). Cerfolio et al. also reported excellent outcomes in 100 patients undergoing RAIL with an anastomotic leak rate of 6%, zero 30-day mortalities, and a 90-day mortality rate of 1%. They compared a stapled intrathoracic anastomosis with a 2-layered hand sewn technique made feasible by the robot. In this series, R0 resection was achieved in all patients, 18 lymph nodes were removed on average, and median operative time was 367 minutes (21). In 2016, Sarkaria et al. reported excellent outcomes in 100 patients undergoing RAMIE with an anastomotic leak rate of 6%, 0% 30-day mortality, and a 90-day mortality rate of 1% (24). In 2017, Okusanya et al. described their Ivor Lewis approach to RAMIE which is their preferred approach to removal of lower esophageal tumors (30). They had initial favorable outcomes with no 30- or 90-day mortality and similar perioperative outcomes including blood loss, anastomotic leak rates, and morbidity compared to MIE.
Retrospective database studies have also been performed comparing MIE and RAMIE. Harbison and colleagues used the American College of Surgeons NSQIP database to compare the two approaches (31). In an analysis of 725 cases (100 RAMIE vs. 625 MIE), there were no differences in short term postoperative adverse outcomes. Similarly, Espinoza-Mercado compared MIE, RAMIE, and OE, using the national cancer database (32). In this study, mortality and readmission rates were similar amongst groups, while the median length of stay was significantly shorter in the MIE and RAMIE groups, compared to the OE group. The authors also found that there was no difference in median overall survival (32).
The ROBOT trial was the first randomized control trial performed by van der Sluis et al. Between January 2012 and 2016, 112 patients with resectable intrathoracic esophageal cancer were randomized to the standard curative treatment of perioperative or preoperative chemoradiation followed by open transthoracic esophagectomy or RAMIE. Post-operative complications including cardiopulmonary occurred less frequently after RAMIE (59% compared to 80%). There was decreased mean post-operative pain using the visual analog scale and improved quality of life score at discharge and at 6 weeks follow up. Both short and long term oncological outcomes were similar with average follow up of 40 months (33,34).
MIE Ivor-Lewis esophagectomy used to be our preferred approach. Recently we transitioned to robotic approach. The authors compared their experience of MIE vs. RAMIE and found the outcomes to be comparable with robotic approach being associated with increased number of lymph node harvested and fewer re-admissions. There were no mortalities or anastomotic leaks. The operative time was around 310 minutes with a median length of stay 7 days in both groups. Overall, RAMIE is a safe, feasible, and potentially advantageous approach to esophagectomy.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ghulam Abbas) for the series “Minimally Invasive Esophageal Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/shc-2019-mies-10). The series “Minimally Invasive Esophageal Surgery” was commissioned by the editorial office without any funding or sponsorship. GA served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Shanghai Chest from June 2019 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Howlader NNA, Krapcho M, Miller D, et al. SEER Cancer Statistics Review. In. based on November 2018 SEER data submission. National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2016/
- Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg 2017;6:131-6. [Crossref] [PubMed]
- Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev 2008;17:352-8. [Crossref] [PubMed]
- Rubenstein JH, Taylor JB. Review Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 2010;32:1222-7. [Crossref] [PubMed]
- Lagergren J, Bergstrom R, Lindgren A, et al. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Drewes AM, et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375-83. [Crossref] [PubMed]
- Torek F, Dubecz A, Schwartz S. Our Surgical Heritage. Ann Thorac Surg 2008;85:1497-9.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393:1948-57. [Crossref] [PubMed]
- Donohoe CL, Reynolds JV. Neoadjuvant treatment of locally advanced esophageal and junctional cancer: the evidence-base, current key questions and clinical trials. J Thorac Dis 2017;9:S697-704. [Crossref] [PubMed]
- Shapiro J, van Lanschot JB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital Volume and Surgical Mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Hess NR, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
- Xie MR, Liu CQ, Guo MF, et al. Short-term outcomes of minimally invasive Ivor-Lewis esophagectomy for esophageal cancer. Ann Thorac Surg 2014;97:1721-7. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Wei B, D'Amico TA. Thoracoscopic versus robotic approaches: advantages and disadvantages. Thorac Surg Clin 2014;24:177-88. vi. [Crossref] [PubMed]
- van Hillegersberg R, Boone J, Draaisma WA, et al. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg Endosc 2006;20:1435-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
- Kernstine KH, DeArmond DT, Shamoun DM, et al. The first series of completely robotic esophagectomies with three-field lymphadenectomy: initial experience. Surg Endosc 2007;21:2285-92. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin 2014;24:211-22. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Grosser R, et al. Attaining Proficiency in Robotic-Assisted Minimally Invasive Esophagectomy While Maximizing Safety During Procedure Development. Innovations (Phila) 2016;11:268-73. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Computer-enhanced robotic telesurgery. Initial experience in foregut surgery. Surg Endosc 2002;16:1790-2. [Crossref] [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus 2013;26:159-66. [Crossref] [PubMed]
- Puntambekar S, Kenawadekar R, Kumar S, et al. Robotic transthoracic esophagectomy. BMC Surg 2015;15:47. [Crossref] [PubMed]
- de la Fuente SG, Weber J, Hoffe SE, et al. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc 2013;27:3339-47. [Crossref] [PubMed]
- Okusanya OT, Hess NR, Luketich JD, et al. Technique of robotic assisted minimally invasive esophagectomy (RAMIE). J Vis Surg 2017;3:116. [Crossref] [PubMed]
- Harbison GJ, Vossler JD, Yim NH, et al. Outcomes of robotic versus non-robotic minimally-invasive esophagectomy for esophageal cancer: An American College of Surgeons NSQIP database analysis. Am J Surg 2019;218:1223-8. [Crossref] [PubMed]
- Espinoza-Mercado F, Imai TA, Borgella JD, et al. Does the Approach Matter? Comparing Survival in Robotic, Minimally Invasive, and Open Esophagectomies. Ann Thorac Surg 2019;107:378-85. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, van der Horst S, et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials 2012;13:230. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled T
Cite this article as: Musgrove K, Spear CR, Kakuturu J, Harris BR, Abbas F, Abdelsattar JM, Abbas G. Robotic assisted minimally invasive esophagectomy: Ivor-Lewis approach. Shanghai Chest 2021;5:21.