
Minimally-invasive surgery for concurrent intralobar pulmonary sequestration and bronchogenic cyst in an adult: a case report
Introduction
Congenital pulmonary anomalies (CPA) constitute a wide spectrum of rare diseases including congenital pulmonary artery malformation, congenital lobar emphysema, pulmonary sequestration and bronchogenic cyst. Their reported overall incidence is around 1 case over 8,000 newborns (1).
The main feature of pulmonary sequestration (PS) is the presence of non-functioning lung tissue, without communication with the airway, receiving an arterial supply from the systemic circulation. PSs are subclassified into extralobar and intralobar types, that have similar histological features but distinct locations; moreover, they have different venous return directed to the systemic (extralobar PS) or pulmonary circulation (intralobar PS) (1). While in case of extralobar PS the dysplastic tissue is isolated from healthy pulmonary parenchyma and surrounded by the pleural layer, intralobar PS, which accounts for about three-quarters of all patients affected by the disease, is located as a part of the lung, usually the left lower lobe (2).
Bronchogenic cyst (BC) is a CPA that arise from the developmental pathway of the foregut endoderm, similar to foregut duplication of the upper gastrointestinal system. BCs do not communicate with the normal airway, and they have usually a mediastinal location. Clinically, in some cases these conditions may be asymptomatic and diagnosed by chance. Instead, postnatal presentation varies from respiratory distress in infancy to chronic cough and recurrent infections in adults. Surgical treatment of these lesions may be required because of the reported association with malignant tumors (1).
The development of prenatal ultrasound (US) and magnetic resonance imaging (MRI) led to an improvement of the prognosis in patients affected by CPAs that could benefit of fetal surgery (1). However, a considerable proportion of them still do not show symptoms related to presence of such anomalies and are diagnosed in the adult age.
While about 50% of patients with extralobar PS show a concurrent presence of other developmental anomalies, the association of intralobar PS with other congenital lesions, such as foregut duplication or BC, is very rare (2). We describe herein the case of an adult patient with an intralobar left lower lobe PS and a posterior mediastinal BC successfully treated by video-assisted thoracic surgery (VATS). The case is presented in accordance with the CARE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-21-27/rc).
Case presentation
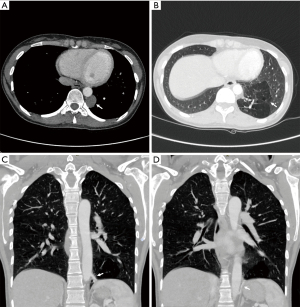
A 29-year-old woman underwent preoperative evaluation for the surgical treatment of a uterine myoma causing anemia. Clinical examination and biochemical investigations were unremarkable. A chest X-ray showed the presence of an opacity in the left posterior mediastinal space. The patient underwent a contrast enhanced chest CT scan that confirmed the presence of a 4 cm-wide rounded cystic lesion with low density in the left posterior mediastinum adjacent to the descending aorta (Figure 1A). Moreover, the imaging showed a hypovascularized parenchymal area in the posterior basal segment of the left lower pulmonary lobe (Figure 1B) with two aberrant arterial branches originating from the lateral side of the descending thoracic aorta (Figure 1C) and from the abdominal aorta proximal to the celiac trunk (Figure 1D), respectively. The cranial aberrant branch was close to a venous segmental pulmonary vessel that was early enhanced after contrast injection with normal size compared to the other enlarged branches of the inferior pulmonary vein. These radiological findings were suspect for an intralobar pulmonary sequestration associated to an intraparenchymal arteriovenous fistula.
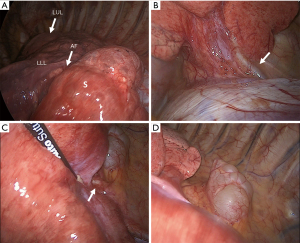
The patient was scheduled for a left video-thoracoscopy with diagnostic and therapeutic intent. Routine preoperative evaluation (EKG, pulmonary function test with DLCO evaluation, arterial blood gas analysis) showed normal results. Surgery was conducted under general anesthesia with double lumen intubation and one lung ventilation through a standard Copenhagen triportal VATS approach. Chest exploration confirmed an area of abnormal dystrophic parenchyma, clearly demarcated from the healthy lung by the presence of an accessory fissure, consistent with a lower intralobar sequestration involving the dorsal basal segment (Figure 2A) with two aberrant arteries, the one originating from the abdominal aorta located at the costophrenic angle (Figure 2B), and the other originating from the descending aorta (Figure 2C). The paravertebral mass consisted in a para-aortic cyst (Figure 2D). The aberrant arteries were isolated and stapled with separate vascular reloads (30 mm Tristaple Endo GIA™ Curved Tip, Medtronic, USA-Ireland) as close as possible to their origin from the aorta, and the sequestration was excised from the functioning lower lobe with a wedge resection. The cyst was dissected from the aortic wall and completely removed. Skin-to-skin operative time was 140 min. There were no intraoperative complications and total blood loss was negligible. The post-operative course was uneventful and the patient was discharged from hospital on the fourth post-operative day.
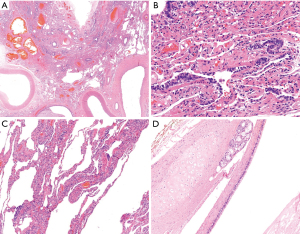
Gross examination of the resected lung confirmed an intralobar sequestration, measuring 5 cm × 6 cm × 4 cm. After section, it presented with many cystic formations, microscopically covered by ciliated cylindrical epithelium, filled by white-yellowish and creamy material and vascularized by ectatic arteries (Figure 3A-3C). The para-aortic mediastinal cystic lesion measured 2.8 cm and consisted of multiple cysts full of mucous solid material. Microscopically, a ciliated cylindrical epithelium was identified, with seromucous glands and cartilage plates in the wall (Figure 3D). These findings led to a final diagnosis of bronchogenic cyst.
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The surgical excision of CPA is recommended because of an intrathoracic mass effect, to reduce the incidence of superinfections, and to prevent the possibility of malignant transformation regardless of age at the time of diagnosis. In fact, a systematic review by Casagrande et al. showed that in the adult population patients with BCs carry a higher risk of later development of primary pulmonary adenocarcinoma. The occurrence of lung cancer has been reported also in case of PS (3).
The concurrent presentation of intralobar pulmonary sequestration and bronchogenic cyst is an unusual condition, which has been to date described in the literature in a limited number of cases (Table 1) (4-10). Most patients were adults between 32 and 49 years of age. Barbut et al. described the case of a female newborn who received a prenatal diagnosis of a left thoracic cyst by US and MRI, and was operated at the age of 5 months with the final diagnosis of a left upper intralobar PS associated with a BC (9). Another female 5-year-old child with an history of pneumonia underwent a left lower lobectomy for an intralobar PS associated with a CPAM, and the concurrent excision of two extrapulmonary BCs (7).
Table 1
| Author, year | Case No. | Gender, age (years) | Symptoms | Diagnostics | Location | Surgical approach | Procedure | Postop complications |
|---|---|---|---|---|---|---|---|---|
| Croyle, 1982 (4) | 1 | M, 49 | Asymptomatic | CT, angiography | LLL | Thoracotomy | Wedge resection | None |
| Grewal, 1994 (5) | 2 | F, 36 | Recurrent pneumonia | CT, angiography, bronchoscopy, barium swallow, MRI | LLL (PS), posterior mediastinum (BC) | Thoracotomy | Lobectomy + resection BC | N/A |
| Wilson, 2005 (6) | 3 | F, 47 | Recurrent pneumonia | CT, bronchoscopy | RLL | N/A | Lobectomy | None |
| 4 | F, 44 | Asymptomatic | CT, bronchoscopy | RLL (PS), mediastinum (BC) | N/A | Lobectomy; BC not removed | None | |
| 5 | F, 39 | Asymptomatic | CT, angiography, bronchoscopy, MRI | RLL | N/A | Lobectomy + staged resection BC | None | |
| Carsin, 2010 (7) | 6 | F, 5 | Pneumonia | CT, US, TEE | LLL (PS), diaphragm and pleura (2 BCs) | N/A | Lobectomy + resection BCs | None |
| Liu, 2010 (8) | 7–10 | 4 patients/47 cases of PS | N/A | CT, angiography, MRI | N/A | N/A | N/A | N/A |
| Barbut, 2011 (9) | 11 | F, 5 months | Antenatal diagnosis | Antenatal US + MRI | LUL | VATS | Lobectomy | None |
| Traibi, 2012 (10) | 12 | F, 32 | Asymptomatic | CT, MRI, TEE | RLL (PS), posterior mediastinum (BC) | VATS | Division of the aberrant artery | Chylothorax requiring reintervention and resection of ischemic PS parenchyma |
| Present case | 13 | F, 29 | Asymptomatic | CT | LLL (PS), posterior mediastinum (BC) | VATS | Wedge resection + resection BC | None |
PS, pulmonary sequestration; BC, bronchogenic cyst; CT, computed tomography; LLL, left lower lobes; MRI, magnetic resonance imaging; RLL, right lower lobes; US, ultrasound; TEE, transesophageal echocardiography; LUL, left upper lobes.
Overall, only few adult patients presented symptoms related to recurrent pulmonary infections (5,6). Cystic lesions were usually incidentally found during clinical evaluation for other reasons. All the authors underline the prominent role of contrast-enhanced CT scan in the diagnostic pathway of intrathoracic cystic lesions, for the identification and location of aberrant vessels (1 or 2) (7), which usually arise from the thoracic descending aorta even if a phrenic or abdominal origin has been reported (8). In selected cases, aortic angiography was used to confirm the diagnosis of PS (4-6,8). Chest and spine MRI, bronchoscopy, contrast study of the upper digestive tract, and transesophageal echocardiography (TEE) have been occasionally performed for differential diagnosis and for surgical planning.
Among the studies reporting the association of intralobar PS and BC, VATS treatment was sporadically reported by the most recent experiences (8-10). Most patients underwent anatomical pulmonary lobectomy with resection of eventual separate extrapulmonary cystic lesions. The incidence of intraoperative complications was low; Liu and colleagues described two cases of massive bleeding caused by the rupture of the feeding artery (8). Almost all the patients showed no postoperative complications, but a case of chylothorax that required reintervention was reported by Traibi et al. (10).
In the last decades, minimally invasive surgery by means of VATS has become the procedure of choice for the treatment of early-stage lung cancer, and it covers an important role in the multimodal approach to more advanced disease. In addition to the well-known advantages of thoracoscopy over thoracotomy (reduced postoperative complications, faster recovery and better cosmetic results, shorter hospital length of stay), VATS demonstrated to be an effective technique also for the concurrent treatment of synchronous pulmonary and mediastinal lesions (11).
Similar results have been outlined by the analysis of the outcome of thoracoscopic resection of PS and BC. Li and colleagues reviewed the data of 110 adult patients with intralobar PS. Compared to those who underwent thoracotomy, patients treated by VATS showed lower blood loss, despite one patient required conversion to open approach caused by the rupture of the aberrant artery of the PS. Moreover, in the VATS group there was a lower incidence of postoperative pleural effusion, and shorter hospital stay (12). In another study, VATS treatment resulted superior to posterolateral thoracotomic approach for the excision of mediastinal BC in terms of reduced blood loss, duration of surgery, chest tube length of stay, and hospitalization (13).
VATS technique has several advantages for the safe and effective treatment of these lesions. In the case reported, the traditional triportal approach allowed a careful dissection of the BC from the aortic wall, and an optimal control for the division the aberrant vessels in the context of the inferior pulmonary ligament. Moreover, the magnified view of thoracoscope allowed a clear identification of the demarcation plane between the healthy pulmonary parenchyma and the PS to be resected, that was improved by the presence of an accessory fissure dividing it from the rest of the left lower lobe.
In the last years, other techniques different from traditional multiportal VATS showed their effectiveness in the surgical treatment of BCAs. The ergonomical drawbacks related to uniportal VATS (U-VATS) have been traditionally considered a major limitation of the technique when facing complex procedures such as the isolation of aberrant vessels of PS. Nevertheless, Kim et al. showed that, in experienced hands, the entire procedure can be carried safely with simple variations of surgical steps, opening new horizons for the dissemination of single port surgery in the treatment of these conditions (14). In a recent case of a young man affected by right lower intralobar PS extending to the left hemithorax with four aberrant arteries, successful complete anatomical resection was achieved employing bilateral robotic surgery approach. According to the authors, the improved dexterity and tridimensional vision of robotics allowed a precise dissection of structures to complete this complex procedure through a minimally invasive technique (15).
Some modifications of the technique have been proposed. A hybrid approach with the combination of preoperative angiographic embolization of the anomalous arterial supply of PS may reduce the risk of bleeding complications during VATS resection, in particular in the presence of vessels originating from the subphrenic aorta (16). Yamanashi et al. described the use of indocyanine green injection and infrared visualization following the division of the arterial and venous branches of the PS to enhance a precise identification of the limits of the affected parenchyma (17). In fact, while most authors suggest to treat patients with intralobar PS by anatomical lobectomy, lung-sparing surgery involving the resection of PS with clear margins is advocated as an alternative choice considering the young age of the affected patients. Indeed, it has been demonstrated that, with the application of a thorough preoperative evaluation and intraoperative management, patients undergoing radical wedge resection for localized intralobar PS benefit of long-term relapse-free survival (18). In this view, a precise identification of the division plane is critical to avoid to leave ischemic parenchyma, as previously described by Traibi et al. (10).
There is not a consensus about the duration of follow up after resection of PS and BC. In a series by Wang et al., among 35 patients overall, 28 underwent VATS treatment for intralobar PS (26 with lobectomy and 2 with wedge resection) showing no recurrence after a median follow up of 57 months (19). The most important factor to prevent relapse is complete resection with clear margins; in fact, late recurrence following not-radical surgery for BC has been described even 24 years after the initial treatment (20).
In conclusion, we presented the thirteenth case ever described of synchronous intralobar PS and mediastinal BC in a young woman. Preoperative contrast-enhanced CT scan provided adequate information for the differential diagnosis and for surgical planning. A VATS approach allowed to successfully treat both lesions minimizing the surgical trauma, and it should be considered the treatment of choice for these diseases in centers with a high volume of VATS operative procedures.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-21-27/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-21-27/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-21-27/coif). AC serves as an unpaid editorial board member of Shanghai Chest from June 2021 to May 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zobel M, Gologorsky R, Lee H, et al. Congenital lung lesions. Semin Pediatr Surg 2019;28:150821. [Crossref] [PubMed]
- Liechty KW, Flake AW. Pulmonary vascular malformations. Semin Pediatr Surg 2008;17:9-16. [Crossref] [PubMed]
- Casagrande A, Pederiva F. Association between Congenital Lung Malformations and Lung Tumors in Children and Adults: A Systematic Review. J Thorac Oncol 2016;11:1837-45. [Crossref] [PubMed]
- Croyle P, Estrera AS. Bronchogenic cyst and intralobar sequestration mimicking thoracic aortic aneurysm. South Med J 1982;75:1267-8. [Crossref] [PubMed]
- Grewal RG, Yip CK. Intralobar pulmonary sequestration and mediastinal bronchogenic cyst. Thorax 1994;49:615-6. [Crossref] [PubMed]
- Wilson RL, Lettieri CJ, Fitzpatrick TM, et al. Intralobular bronchopulmonary sequestrations associated with bronchogenic cysts. Respir Med 2005;99:508-10. [Crossref] [PubMed]
- Carsin A, Mely L, Chrestian MA, et al. Association of three different congenital malformations in a same pulmonary lobe in a 5-year-old girl. Pediatr Pulmonol 2010;45:832-5. [Crossref] [PubMed]
- Liu HS, Li SQ, Qin YZ, et al. Surgical treatment of intralobar pulmonary sequestration. Chin Med Sci J 2010;25:53-6. [Crossref] [PubMed]
- Barbut J, Fernandez C, Blanc F, et al. Pulmonary sequestration of the left upper lobe associated with a bronchogenic cyst: case report of an exceptional association. Pediatr Pulmonol 2011;46:509-11. [Crossref] [PubMed]
- Traibi A, Strauss C, Validire P, et al. Intralobar pulmonary sequestration associated with bronchogenic cyst in adult. Asian Cardiovasc Thorac Ann 2012;20:597-9. [Crossref] [PubMed]
- Zeng L, Zhuang R, Tu Z. Simultaneous uniportal video-assisted thoracic surgery for pulmonary nodules and synchronous mediastinal lesions. Wideochir Inne Tech Maloinwazyjne 2021;16:390-6. [Crossref] [PubMed]
- Li Q, Xie D, Sihoe A, et al. Video-assisted thoracic surgery is associated with better short-term outcomes than open thoracotomy in adult patients with intralobar pulmonary sequestration. Interact Cardiovasc Thorac Surg 2018;26:284-7. [Crossref] [PubMed]
- Guo C, Mei J, Liu C, et al. Video-assisted thoracic surgery compared with posterolateral thoracotomy for mediastinal bronchogenic cysts in adult patients. J Thorac Dis 2016;8:2504-11. [Crossref] [PubMed]
- Kim CW, Kim DH. Single-incision video-assisted thoracic surgery lobectomy in the treatment of adult communicating bronchopulmonary foregut malformation with large aberrant artery. J Thorac Dis 2016;8:E148-51. [PubMed]
- Dagorno C, Sarsam M, Brun AL, et al. A Horseshoe Intralobar Lung Sequestration Resection by Bilateral Robot-Assisted Surgery. Ann Thorac Surg 2022;113:e95-7. [Crossref] [PubMed]
- Grossi W, Londero F, Vit A, et al. Hybrid minimally invasive treatment of intralobar pulmonary sequestration: a single-centre experience. Interact Cardiovasc Thorac Surg 2022;34:255-7. [Crossref] [PubMed]
- Yamanashi K, Okumura N, Nakazono C, et al. Surgery for Intralobar Pulmonary Sequestration Using Indocyanine Green Fluorescence Navigation: A Case Report. Semin Thorac Cardiovasc Surg 2018;30:122-4. [Crossref] [PubMed]
- Sakuma T, Sugita M, Sagawa M, et al. Video-assisted thoracoscopic wedge resection for pulmonary sequestration. Ann Thorac Surg 2004;78:1844-5. [Crossref] [PubMed]
- Wang S, Li Y, Wang J. Video-Assisted Thoracoscopic Surgery for Pulmonary Sequestrations: Series of 35 Consecutive Patients in a Single Center. Thorac Cardiovasc Surg 2019;67:73-8. [Crossref] [PubMed]
- Gharagozloo F, Dausmann MJ, McReynolds SD, et al. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest 1995;108:880-3. [Crossref] [PubMed]
Cite this article as: Muriana P, Negri G, Giuliani M, Filipello F, Arrigoni G, Carretta A. Minimally-invasive surgery for concurrent intralobar pulmonary sequestration and bronchogenic cyst in an adult: a case report. Shanghai Chest 2022;6:18.


