
Spontaneous resolution of metastatic thymoma with prednisone in a patient with juvenile myasthenia gravis: a case report
Introduction
Thymomas are rare in children and when present in patients with juvenile myasthenia gravis (JMG), often carry a poor prognosis (1,2). Studies suggest thymomas occurs in about 1–2% of JMG patients, far less than in the adult myasthenia population (10–30%) (3-5). Thymomas themselves are often associated with paraneoplastic syndromes, with myasthenia gravis occurring over 50% of the time, though this data is limited to adults (6). However, the benefit of thymectomy is unclear in children given the lack of rigorous clinical data in young patients.
Additionally, spontaneous remission of JMG has been reported in less than 40 patients, though the overwhelming majority had mild disease-severity or bulbar predominant myasthenia (7). Here, we present a patient with stage IVa type B2 thymoma and JMG who underwent an extended radical parietal and visceral pleurectomy and decortication and total thymectomy. His thoracic tumor burden continued to progress and his disease course was complicated by myasthenic crisis. However he unexpectedly had spontaneous resolution of his large tumor burden with prednisone. There are scattered reports of metastatic thymoma in adult MG improving with high dose steroids (8-10). However, invasive metastatic thymomas are extremely rare in the pediatric population and have not been well characterized. In this report, we describe a rare case of severe JMG and metastatic thymoma that responded to steroid therapy, highlighting the potential role of corticosteroids for JMG and thymoma refractory to thymectomy. We present the following case in accordance with the CARE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-22-15/rc).
Case presentation
A school-aged boy presented to the clinic with easy fatiguability. He was also noted to have drooping of his left eyelid and intermittent slurred speech. His symptoms were thought to generally worsen over the course of the day. Following a thorough evaluation, he was found to have positive anti-acetylcholine receptor and anti-striated muscle antibody serologies, consistent with a diagnosis of juvenile myasthenia gravis. He was started on pyridostigmine and underwent a chest computed tomography (CT) scan, which showed a 4.9 cm × 4.5 cm × 11.7 cm partially calcified anterior mediastinal mass, as well as multiple lobulated pleural masses (Figure 1). Subsequent positron emission tomography revealed diffuse fluorodeoxyglucose (FDG)-avid uptake by the mediastinal mass, several left anterior costophrenic angle lymph nodes, and pleural masses. Pleural biopsy shortly after revealed an invasive Type B2 thymoma, consistent with Masaoka stage IVa disease given the presence of pleural metastases. Corticosteroid treatment was deferred given the patient’s progressive dyspnea and fatigue, creating a need for more aggressive treatment. Instead, he received several courses of intravenous immunoglobulin (IVIG) and four cycles of neoadjuvant cyclophosphamide, doxorubicin, and cisplatin with no reduction in tumor size.
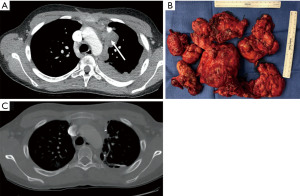
He then underwent a successful left thoracotomy with total thymectomy, extended radical parietal and visceral pleurectomy, and decortication, diaphragm resection and reconstruction, and partial pericardial resection (Figure 1). Diaphragm reconstruction was accomplished with a 20 cm × 30 cm StratticeTM biologic mesh. Several left sided chest tubes were placed and the patient was admitted to the pediatric intensive care unit for post-operative monitoring. His hospital course was overall uncomplicated, as he recovered well and was discharged home on pyridostigmine maintenance therapy. He did not receive corticosteroids on discharge. Final pathology was consistent with stage IVa type B2 thymoma, negative lymph nodes, and positive microscopic margins (R1 resection).
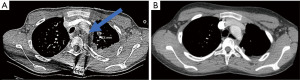
The patient however developed tumor recurrence within one year, as serial follow-up imaging showed disease progression with tumor encasement of mediastinal structures (Figure 2A). He was started on a course of sunitinib without reduction in tumor burden, and this medication was stopped. Following a hospitalization for acute myasthenic crisis, he was started on 60 milligrams prednisone daily with a plan for palliative debulking. His prognosis appeared very poor and the goals of future treatment shifted to prioritize symptom control. Unexpectedly, repeat CT scan two months later showed near complete resolution of his pleural disease (Figure 2B). His prednisone was gradually tapered and he additionally received several doses of rituximab. Notably, his thoracic tumor burden increased when prednisone doses fell below 10 milligrams, necessitating higher doses which were associated with tumor size reduction. He has overall tolerated his prednisone regimen, although experienced a nearly 20% increase in his weight, as well as gastroesophageal reflux that has been controlled with famotidine. He remains on maintenance prednisone with stable tumor burden on surveillance imaging.
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian patients for publication of this case report and accompanying images. Due to the retrospective nature of this case report and since no patient identifiers have been provided, the Institutional Review Board of Kaiser Permanente Northern California does not require approval for this case report. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Juvenile myasthenia gravis is an autoimmune condition affecting individuals under the age of 18 (11). First line therapy for JMG, similar to adult MG, involves pyridostigmine, an acetylcholinesterase inhibitor (11). Additional immunomodulatory agents, including but not limited to corticosteroids, steroid-sparing agents, and intravenous immunoglobulin are often adjunctive pharmaceutical therapies, though there is no clear guidance on the use of these treatments in the pediatric population (11). Chest imaging is part of the standard work up for myasthenia given the strong association with thymomas and its resulting impact of further management.
Thymomas comprise less than one percent of mediastinal tumors in children (12). Type B2 thymomas, as in this patient, are moderately aggressive. In adult patients, B2 thymomas have been associated with better survival than some thymic malignancies, including thymic carcinoma (13). However, the presence of paraneoplastic syndromes, such as myasthenia gravis, in patients with thymomas is associated with decreased survival rates, though these studies again are limited to the adult population (2,6).
The rare incidence of thymomas in children has made it difficult to prognosticate at the time of diagnosis. Expectedly, there is no consensus on thymoma management in children, and published treatment strategies have been adapted from thymoma guidelines in adults. The limited data on thymomas in the pediatric population suggests younger patients more often have later stage disease at the time of diagnosis (12). Furthermore, the incidence of associated juvenile myasthenia gravis is not well established, but appears to occur less frequently than in adults (14).
In general, the presence of a thymoma is an absolute indication for thymectomy. This is rooted in the theory that the thymus may be the origin of plasma cells producing autoantibodies against acetylcholine (15). Numerous studies have shown favorable disease responses post-thymectomy in adults. On the other hand, the benefit of thymectomy in the pediatric population remains unclear. Early thymectomy, which is defined within two years of disease onset, is advised for patients with generalized myasthenic disease, including but not limited to decreased respiratory capacity and bulbar symptoms (16). Thymectomy can also be considered prior to immunosuppressive therapies if a child’s symptoms are stable, in an effort to avoid the post-operative infection risk associated with steroids (16). One of the most comprehensive systemic reviews showed that of 488 children with juvenile myasthenia gravis who underwent thymectomy, 70% had reduction in disease severity (17). Furthermore, the adaptation of minimally invasive thoracoscopic approaches has yielded favorable safety data compared to open surgery, although disease outcomes appear similar with both approaches (18,19). In this case, despite aggressive debulking, this patient continued to have disease progression post-thymectomy, possibly owing to his large tumor burden pre-operatively that did not respond to neoadjuvant chemotherapy.
Studies have suggested that children with JMG have higher rates than adults with MG of spontaneous remission without surgery or immunosuppressive therapies (14,20-22). However, this is difficult to reconcile with concurrent evidence of partial or even complete response after thymectomy (17,18). The patient in this case, however, had progression of his dyspnea and fatigue soon after his diagnosis, which along with significant thoracic tumor burden, informed the decision to proceed with surgery.
The mechanism of spontaneous remission in myasthenia gravis is not understood. In vivo studies have suggested that there may be innate adaptions to the neuromuscular junction that can occur over time that cause decreased binding of acetylcholine receptor antibodies (23). There is no evidence that immunotherapies augment this change. However, several studies have concluded that certain patient characteristics of JMG make one more likely to achieve spontaneous remission. These include mild disease, young age of onset (pre-adolescent), and possibly ocular-predominant MG (24). While this patient ultimately developed recurrence post-thymectomy, it is unclear if his debulking impacted his eventual spontaneous remission once prednisone was started, as spontaneous remission in the minimally-invasive thymectomy era has not been well characterized in JMG. Interestingly in adults, the combination of thymectomy and alternate day prednisone has been shown to have superior outcomes compared to prednisone alone, suggesting an augmenting effect (25).
This case report serves to highlight the potential beneficial role of high dose steroids administered over long tapers in children with juvenile myasthenia gravis and refractory thymomas. Despite higher incidence of spontaneous remission, clear guidelines are nonetheless required for this multifaceted condition. The role of both pharmaceutical and surgical interventions remains to be further defined for this patient population and reports such as this will add to existing, limited data on refractory JMG. Given the rarity of thymomas in the pediatric population, continued characterization of the spectrum of clinical presentations and outcomes is crucial for developing treatment modalities for these patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-22-15/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-15/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-15/coif). Jeffrey B. Velotta serves as an unpaid editorial board member of Shanghai Chest from March 2022 to February 2024. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Masaoka A. Staging system of thymoma. J Thorac Oncol 2010;5:S304-12. [Crossref] [PubMed]
- Saha S, Suhani S, Basak A, et al. Pediatric thymoma with a difference: report of a case and review of literature. J Surg Tech Case Rep 2014;6:64-6. [Crossref] [PubMed]
- Chou CC, Su IC, Chou IJ, et al. Correlation of anti-acetylcholine receptor antibody levels and long-term outcomes of juvenile myasthenia gravis in Taiwan: a case control study. BMC Neurol 2019;19:170. [Crossref] [PubMed]
- Gui M, Luo X, Lin J, et al. Long-term outcome of 424 childhood-onset myasthenia gravis patients. J Neurol 2015;262:823-30. [Crossref] [PubMed]
- Mao ZF, Mo XA, Qin C, et al. Incidence of thymoma in myasthenia gravis: a systematic review. J Clin Neurol 2012;8:161-9. [Crossref] [PubMed]
- Kumar R. Myasthenia gravis and thymic neoplasms: A brief review. World J Clin Cases 2015;3:980-3. [Crossref] [PubMed]
- Arroyo HA, Torres AR. Spontaneous remission in juvenile myasthenia gravis: A cohort of 13 cases and review of the literature. Neuromuscul Disord 2022;32:213-9. [Crossref] [PubMed]
- Wrona E, Dębska-Szmich S, Pastuszka M, et al. Remission of Thymoma on Steroid Therapy in a Patient With Atypical Thymoma-Associated Multiorgan Autoimmunity: A Case Report and Literature Review. Front Immunol 2021;12:584703. [Crossref] [PubMed]
- Qi G, Liu P, Dong H, et al. Metastatic Thymoma-Associated Myasthenia Gravis: Favorable Response to Steroid Pulse Therapy Plus Immunosuppressive Agent. Med Sci Monit 2017;23:1217-23. [Crossref] [PubMed]
- Kirkove C, Berghmans J, Noel H, et al. Dramatic response of recurrent invasive thymoma to high doses of corticosteroids. Clin Oncol (R Coll Radiol) 1992;4:64-6. [Crossref] [PubMed]
- O'Connell K, Ramdas S, Palace J. Management of Juvenile Myasthenia Gravis. Front Neurol 2020;11:743. [Crossref] [PubMed]
- Liang X, Lovell MA, Capocelli KE, et al. Thymoma in children: report of 2 cases and review of the literature. Pediatr Dev Pathol 2010;13:202-8. [Crossref] [PubMed]
- Song Z, Jin X, Zhang Y. Treatment and prognosis of type B2 thymoma. World J Surg Oncol 2014;12:291. [Crossref] [PubMed]
- Castro D, Derisavifard S, Anderson M, et al. Juvenile myasthenia gravis: a twenty-year experience. J Clin Neuromuscul Dis 2013;14:95-102. [Crossref] [PubMed]
- Fichtner ML, Jiang R, Bourke A, et al. Autoimmune Pathology in Myasthenia Gravis Disease Subtypes Is Governed by Divergent Mechanisms of Immunopathology. Front Immunol 2020;11:776. [Crossref] [PubMed]
- Munot P, Robb SA, Niks EH, et al. 242nd ENMC International Workshop: Diagnosis and management of juvenile myasthenia gravis Hoofddorp, the Netherlands, 1-3 March 2019. Neuromuscul Disord 2020;30:254-64. [Crossref] [PubMed]
- Madenci AL, Li GZ, Weil BR, et al. The role of thymectomy in the treatment of juvenile myasthenia gravis: a systematic review. Pediatr Surg Int 2017;33:683-94. [Crossref] [PubMed]
- Derderian SC, Potter DD, Bansal S, et al. Open versus thoracoscopic thymectomy for juvenile myasthenia gravis. J Pediatr Surg 2020;55:1850-3. [Crossref] [PubMed]
- Goldstein SD, Culbertson NT, Garrett D, et al. Thymectomy for myasthenia gravis in children: a comparison of open and thoracoscopic approaches. J Pediatr Surg 2015;50:92-7. [Crossref] [PubMed]
- Ashraf VV, Taly AB, Veerendrakumar M, et al. Myasthenia gravis in children: a longitudinal study. Acta Neurol Scand 2006;114:119-23. [Crossref] [PubMed]
- Popperud TH, Boldingh MI, Rasmussen M, et al. Juvenile myasthenia gravis in Norway: Clinical characteristics, treatment, and long-term outcome in a nationwide population-based cohort. Eur J Paediatr Neurol 2017;21:707-14. [Crossref] [PubMed]
- Vecchio D, Ramdas S, Munot P, et al. Paediatric myasthenia gravis: Prognostic factors for drug free remission. Neuromuscul Disord 2020;30:120-7. [Crossref] [PubMed]
- Phillips WD, Vincent A. Pathogenesis of myasthenia gravis: update on disease types, models, and mechanisms. F1000Res 2016;5:eF1000 Faculty Rev 1513;
- Mao Z, Hu X, Lu Z, et al. Prognostic factors of remission in myasthenia gravis after thymectomy. Eur J Cardiothorac Surg 2015;48:18-24. [Crossref] [PubMed]
- Wolfe GI, Kaminski HJ, Aban IB, et al. Randomized Trial of Thymectomy in Myasthenia Gravis. N Engl J Med 2016;375:511-22. [Crossref] [PubMed]
Cite this article as: Ramesh N, Velotta JB. Spontaneous resolution of metastatic thymoma with prednisone in a patient with juvenile myasthenia gravis: a case report. Shanghai Chest 2022;6:27.


