
T4 spine and thoracic wall invasive lung cancer treatment, case report
Introduction
Lung cancer is a disease with a high mortality rate and an impact on the quality of life of our patients, being the second most common cancer and the first cause of death from cancer in the world; which forces us to make a constant effort in early diagnosis and curative treatment. However, locally advanced cancer represents a surgical challenge due the possibility of adding years with quality of life without the comorbidities associated with the procedure (1,2). For this reason, various protocols and techniques have been developed with the aim of performing complete resections with minimal residual disease in spite of the great technical difficulty. We present the following case in accordance with the CARE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-21-35/rc).
Case presentation
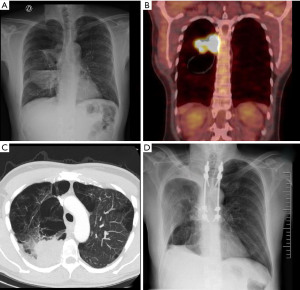
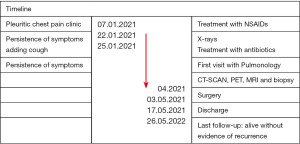
We present a 50-year-old patient, former smoker on varenicline, with a history of pulmonary emphysema diagnosed 20 years ago, who consulted for 2 weeks of evolution of right pleuritic pain that did not resolve with non-steroidal anti-inflammatory drugs (NSAIDs); because it adds cough as a symptom the patient consults in the emergency room where the physical examination was normal, eupneic, an analysis is performed without pathological findings, a negative test for SARSCoV-2 and a chest radiograph (Figure 1A) that shows right pleural effusion with air-fluid level in the apical segment of the right lower lobe suggestive of “abscess/empyema” initiating antibiotic therapy. After completing the treatment without clinical or radiological improvement, it was decided to carry out a computed tomography scanner (CT-SCAN) that describes a sub-pleural spiculated mass in the posterior segment of the RUL with a diameter of 63 mm accompanied by a cyst with hydro-pneumothorax, with pleural and mediastinal contact and a 15 mm hilar adenopathy. A more extensive study was carried out using 18 fluorodeoxyglucose-positron emission tomography/CT (18FDG-PET-CT) (Figure 1B) that showed an intense uptake of the mass standardized uptake value (SUV-max) of 13.1 and hilar adenopathy (SUV-max 2.6) with an no metabolic behavior of the cyst; a magnetic resonance imaging (MRI) that reports: a solid mass with spiculated edges accompanied by cyst with content, suggesting the differential diagnosis between cystic adenomatoid malformation or bronchogenic cyst; relevant for the association with malignant transformation; finally the CT (Figure 1C) showing vertebral infiltration at the level of T6 and the costovertebral joint. For the histological diagnosis, a CT-guided fine needle aspiration (CT-FNA) puncture was performed, reporting: undifferentiated carcinoma with necrosis and positivity for CK CAM5.2. Thus, it is oriented as a cT4N1M0 non-small cell carcinoma of lung origin. Due to the size, local infiltration and lymph node involvement, it was decided to perform a video mediastinoscopy for staging, which ruled out mediastinal lymph node involvement. After performing pulmonary function tests and evaluating it by the multidisciplinary oncology committee, surgery was decided. The intervention was then divided into two stages. The first one carried out by a neurosurgery team that by posterior approach in the prone position, performed the percutaneous fixation of vertebral bodies T4 to T9 left and T4-T5, T8-T9 right. Later, a laminectomy and hemi-vertebrectomy of T6 and T7 under radiological control was performed. In a second stage, with the patient in the lateral decubitus position, the thoracic surgery team, by means of a right posterolateral thoracotomy, performed the in-bloc resection of the right upper lobe (RUL), the 5th to 8th posterior costal arches and the previously prepared T6-T7 hemivertebrae. The patient was transferred to an intensive care unit, being extubated in the first 24 hours, without requiring vasoactive drugs and started oral tolerance 24 hours after surgery. After optimizing pain control, the patient was transferred to a conventional hospital ward where began to walk with a girdle and started respiratory rehabilitation; the drains were removed on the 5th and 13th postoperative day and was discharged on the 14th day (Figure 1D). The pathological analysis confirms a poorly differentiated large cell carcinoma with diffuse infiltration of the spongy bone tissue pT4N0M0 stage IIIA R1, the immunohistochemistry is positive for PD-L1 with mutated TP53. The patient is autonomous and maintains a good quality of life while completing cancer treatment through chemotherapy and radiotherapy without evidence of recurrence even a year after surgery (Figure 2).
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Vertebral resection surgery for direct invasion of lung cancer is technically possible and safe (2-4) meeting resectability criteria. Prognostic factors after surgical intervention for lung neoplasms that invade the wall or spine are the absence of nodules (N) at diagnosis (5,6), complete resection (4,5), and response to chemotherapy (6-10). When there is bone infiltration, it is not possible to evaluate the microscopic resection margins intraoperatively. For this reason, even in centers with extensive experience, in 15–20% of cases the margins are affected in the final pathological analysis (1-4), requiring completion of the oncological treatment by radiotherapy (7,8). Another controversial point in these cases is the induction strategy. There are multiple clinical trials that have randomized patients between isolated surgery vs. chemotherapy induction followed by surgery. Some of these have standardized certain parameters for the induction of patients prior to surgery, however, others practice it in all cases. Whether or not induction is applied, the need to obtain disease-free margins are the same, the application of adjuvant treatment being essential regardless of whether or not neoadjuvant treatment was used (3). The neoadjuvant treatment has been shown to improve disease progression-free in certain groups (superior sulcus tumors) without showing statistically significant improvements in long-term survival in all advanced-stage tumors (11). Studies on long-term results such as the one by Fadel et al. (4) have shown that there was a non-significant increase in local recurrence in patients with positive resection margins, considering that adjuvant treatment and radiotherapy were performed in all cases. Some groups prefer to perform surgery to assess the margins (7,8) while others defend better results after inductions with complete response. In our case, the patient was evaluated by a thoracic tumor board and since it is not a tumor of the superior sulcus, the original sample was reported as indeterminate carcinoma and it was a locally advanced tumor, surgery was decided without prior induction therapy. In our opinion, vertebral infiltration should not be considered a contraindication for surgery. In selected cases, in experienced centers, patients can benefit from a surgical approach, demonstrating promising survival results (5,6).
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-21-35/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-21-35/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-21-35/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamanashi K, Menju T, Hamaji M, et al. Prognostic factors related to postoperative survival in the newly classified clinical T4 lung cancer. Eur J Cardiothorac Surg 2020;57:754-61. [PubMed]
- Gomez-Caro A, Glorion M, Fabre D, et al. F-142, Surgical approaches for en-bloc resection of malignancies involving the thoracic spine. Interactive CardioVascular and Thoracic Surgery 2015;21:S39. [Crossref]
- Schirren J, Dönges T, Melzer M, et al. En bloc resection of non-small-cell lung cancer invading the spine. Eur J Cardiothorac Surg 2011;40:647-54. [Crossref] [PubMed]
- Fadel E, Missenard G, Court C, et al. Long-term outcomes of en bloc resection of non-small cell lung cancer invading the thoracic inlet and spine. Ann Thorac Surg 2011;92:1024-30; discussion 1030. [Crossref] [PubMed]
- Anraku M, Waddell TK, de Perrot M, et al. Induction chemoradiotherapy facilitates radical resection of T4 non-small cell lung cancer invading the spine. J Thorac Cardiovasc Surg 2009;137:441-7.e1. [Crossref] [PubMed]
- Chadeyras JB, Mazel C, Grunenwald D. Vertebral en bloc resection for lung cancer: twelve years' experience. Ann Chir 2006;131:616-22. [Crossref] [PubMed]
- Matsuoka H, Nishio W, Okada M, et al. Resection of chest wall invasion in patients with non-small cell lung cancer. Eur J Cardiothorac Surg 2004;26:1200-4. [Crossref] [PubMed]
- Lanuti M. Surgical Management of Lung Cancer Involving the Chest Wall. Thorac Surg Clin 2017;27:195-9. [Crossref] [PubMed]
- Kumar N, Malik PS, Bharati SJ, et al. Primary lung cancer with chest wall involvement: Outcomes of a multimodality management approach. Lung India 2021;38:338-42. [Crossref] [PubMed]
- Liang H, Yang C, Gonzalez-Rivas D, et al. Sleeve lobectomy after neoadjuvant chemoimmunotherapy/chemotherapy for local advanced non-small cell lung cancer. Transl Lung Cancer Res 2021;10:143-55. [Crossref] [PubMed]
- Majem M, Hernández-Hernández J, Hernando-Trancho F, et al. Multidisciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clin Transl Oncol 2020;22:21-36. [Crossref] [PubMed]
Cite this article as: Quiroga NI, Boada M, Guzmán R, Poblete J, Molins L. T4 spine and thoracic wall invasive lung cancer treatment, case report. Shanghai Chest 2022;6:26.


