
Additional pleural painting with iodopovidone during video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax: an observational, single-center retrospective analysis
Introduction
Primary spontaneous pneumothorax (PSP) is a relatively frequent clinical entity with a high incidence in the young population, generally accompanied by typical symptoms and well-known predisposing factors (1-3).
Following the rupture of subpleural blebs or bullae, located primarily in the upper lobes and apex (4), the management of PSP focuses on cessation of air leak (AL) and prevention of recurrences. Treatment approaches may vary depending on the severity of symptoms and persistence of AL (5).
Currently, the combination of bullectomy and pleurodesis is the best available treatment for recurrent or complicated PSP cases (6-8). Video-assisted thoracic surgery (VATS) approach is widely recognized as a safe surgical technique for treating recurrent or complicated PSP with a lower impact on respiratory function and postoperative pain, and reduced hospital stay than open thoracotomy (9). Surgical or chemical pleurodesis may be an adjunctive therapy after drainage or surgical treatment to prevent recurrent episodes (10). Talc is the most frequently used agent because it is inexpensive and effective (11).
Performing talc pleurodesis instead of surgical pleurodesis (e.g., pleural abrasion, pleurectomy, pleural tenting) has shown several short-term complications and long-term consequences, such as respiratory function and oncological problems, especially in the young population. However, it is less painful in the postoperative course and reduces bleeding risk compared to the surgical options. In terms of prevention of recurrence, the two procedures showed equivalent outcomes. Talc pleurodesis offers excellent results (although data from adequate comparative studies are lacking), but it should be avoided in a young population (12,13).
Nevertheless, several alternatives to talc have been explored in recent years. For example, intrapleural iodopovidone demonstrated its efficacy in recurrent pleural effusion (14) and pneumothorax (15,16). However, there is a lack of studies that firmly assessed the concentration of iodopovidone or specific instructions for its use within the pleural cavity. Primarily, the authors report its injection into the pleural space without mentioning the technic of its distribution during VATS lung resection.
Based on these considerations, we aimed to validate a new VATS technique by painting iodopovidone solution on the parietal pleura after the resection of bullous areas. With the present retrospective observational study, we assessed the efficacy and safety of this intraoperative treatment in young patients compared to talc poudrage in the surgical treatment of PSP. We present the following article in accordance with the STROBE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-22-14/rc).
Methods
This observational retrospective study was approved by the Institutional Review Board of Cagliari (Reference Ethics Committee No. PG/2018/17166) and conducted according to the Helsinki Declaration (as revised in 2013). Informed written consent was obtained from all the patients. We analyzed data of 100 consecutive PSP patients who underwent VATS bullectomy and pleurodesis, either with talc or idopovidone, from July 2012 to February 2020. Eligibility criteria included age ≤40, clinical and radiological findings of a second episode of spontaneous pneumothorax, or a recurrence after previous surgery. Patients with severe emphysema or deemed eligible for lung volume reduction surgery (e.g., vanishing lung syndrome) were excluded from this study. VATS was indicated according to the international guidelines for the surgical management of spontaneous pneumothorax (17).
For each patient, we collected the following data: age, gender, smoking habit, surgical indication, side of surgery, number of thoracoscopic ports, lung resection site, presence of pleural adhesions, pleurodesis technique, operative time, chest drain duration, fluid output, presence of persistent AL (pAL) intended as lasting ≥5 days, residual pneumothorax (intended as apex to cupola distance ≥2 cm) 48 hours after surgery, postoperative complications according to Clavien-Dindo classification (18), the visual analogue scale of pain (VAS) (19), postoperative length of stay (pLOS), ipsilateral pneumothorax recurrence during one-year postoperative follow-up (FU).
Pre-operative workup for all patients included chest High Resolution - CT study (HRCT), cardiology assessment, and anaesthesiologists’ evaluation.
All the procedures were performed under general anaesthesia and single-lung ventilation. The patients were placed in lateral decubitus for proper surgical side exposure. A VATS bullectomy or blebectomy was performed using a standard automated stapling technique, followed by accurate pneumostasis testing. The choice between single-port or multiport thoracoscopic approach depended on each surgeon’s preference and patients’ acceptance. The additional treatment of intraoperative pleurodesis was offered based on patient acceptance. Thus, it was performed based on the availability of sclerosing agents and the history of any allergy reaction to them.
Iodopovidone painting (IP) was defined as the injection of 100 mL of 2% iodopovidone solution in the chest cavity and its distribution over the parietal pleura using a soaked sponge (Figure 1), without performing any pleural scraping and avoiding spread over the mediastinal surface. Talc poudrage (TP) was defined as the insufflation of 2–3 g sterile talc throughout one of the VATS accesses with a commercial talc spray atomizer, ensuring homogenous distribution within the pleural space. Based on the chemical agent used for pleurodesis, two different groups of patients were therefore created: IP group and TP group.
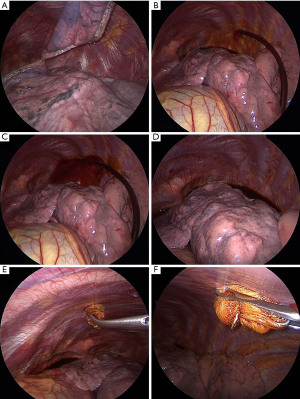
In order to prevent postoperative pain, different analgesia approaches were adopted, such as an elastomeric intravenous pump (EIVP) with non-steroidal anti-inflammatory drugs (NSAID) and morphine or a local anesthetic (bupivacaine 0.25%) infusion through a para-vertebral catheter. Supplemental NSAIDs or paracetamol were administered at patients’ requests, if necessary. Analgesia methods were based on the anesthesiologist’s experience and patients’ consent.
A 28 French chest tube (ChT) was routinely placed at the end of the procedure. Forced negative pressure up to −40 cmH2O was the routine for at least 48 hours after surgery and kept in case of AL or radiological signs of persistent pneumothorax. Chest radiographies (CR) on postoperative day (POD) 1 and 2 were the standard, followed by additional ones according to clinical findings and surgeon’s instructions. Drain output and AL were recorded daily until ChT removal, which was performed at the cessation of AL and <3 mL/kg/2 h of serous ChT output, and after radiographic evidence of a satisfactory lung re-expansion. Patient discharge was declared after ChT discontinuation, followed by CR verification. One week after surgery, follow-up provided an early clinical and radiological assessment. Follow-up was extended to 1 year after surgery, with complete clinical and radiological follow-up after one, three, and six months.
The study’s primary outcome was the recurrence of PNX, defined as hospital readmission due to radiological evidence of relapse during follow-up. In addition, secondary outcomes such as duration of surgery, postoperative complications, the persistence of pneumothorax, patients’ pain report, drain management, and pLOS were evaluated.
Statistical analysis
Data quality was assessed in relation to the accuracy, completeness, and missing information to address potential bias sources. Descriptive analyses were performed to evaluate the baseline distribution of each variable for all patients undergoing VATS bullectomy/blebectomy and additional pleurodesis. Qualitative variables were described with absolute and relative (percentages) frequencies, whereas quantitative variables were summarized with means (standard deviations, SD) or medians [interquartile ranges, IQR] in the case of parametric and non-parametric distribution, respectively. Shapiro-Wilk test was used to verify the normal distribution. Qualitative variables were compared using chi-square or Fisher’s exact tests, whereas ANOVA and Kruskal-Wallis tests were used to compare parametric and non-parametric quantitative variables, respectively. Linear regression analysis was performed to assess the relationship between demographic and clinical variables and quantitative outcomes. Logistic regression analysis was carried out to assess the relationship between IP and TP groups, chest tube serum output, and residual CR pleural space 48 hours after surgery. A two-tailed P value less than 0.05 was considered statistically significant. Only the most relevant results have been reported in this study. StataCorp. 2021 Stata Statistical Software: Release 17 (College Station, TX: StataCorp LLC) was used for the analysis.
Results
One hundred consecutive patients from a juvenile cohort underwent thoracoscopic intervention for surgical treatment of PSP at a reference Hospital in Cagliari, Sardinia, Italy (Table 1).
Table 1
| Characteristics | Iodopovidone (n=50) | Talc poudrage (n=50) | Total (n=100) | P value |
|---|---|---|---|---|
| Median [IQR] age, years | 26 [20–31] | 27 [19–32] | 26.5 [20–31.5] | 0.99 |
| Female, n (%) | 10 (20.0) | 15 (30.0) | 25 (25.0) | |
| Side, n (%) | ||||
| Left | 22 (44.0) | 22 (44.0) | 44 (44.0) | 1.00 |
| Right | 28 (56.0) | 28 (56.0) | 56 (56.0) | |
| Type, n (%) | ||||
| PSP-recurrence | 45 (90.0) | 43 (86.0) | 88 (88.0) | 0.54 |
| PSP-controlateral | 5 (10.0) | 7 (14.0) | 12 (12.0) | |
| Active PNX, n (%) | 32 (64.0) | 36 (72.0) | 68 (68.0) | 0.39 |
| Pathological report, n (%) | ||||
| Apex bullae UL | 40 (80.0) | 44 (88.0) | 84 (84.0) | 0.20 |
| Apex posterior UL bullae | 3 (6.0) | 1 (2.0) | 4 (4.0) | |
| Anterior bullae UL | 1 (2.0) | 0 (0.0) | 1 (1.0) | |
| Bullae LL | 3 (6.0) | 0 (0.0) | 3 (3.0) | |
| Apex bullae UL + other | 1 (2.0) | 3 (6.0) | 4 (4.0) | |
| Bullae LL + ML | 1 (2.0) | 0 (0.0) | 1 (1.0) | |
| Apex bullae UL catamenial | 0 (0.0) | 1 (2.0) | 1 (1.0) | |
| Bullae ML | 0 (0.0) | 1 (2.0) | 1 (1.0) | |
| Apex bullae UL+ML | 1 (2.0) | 0 (0.0) | 1 (1.0) | |
| Pre-operative chest CT scan (n=99), n (%) | 26 (52.0) | 29 (59.2) | 55 (55.6) | |
| Adherence (n=99), n (%) | 19 (38.0) | 17 (34.7) | 36 (36.4) | |
| Ports, n (%) | ||||
| 1 | 26 (52.0) | 44 (88.0) | 70 (70.0) | <0.0001* |
| 2 | 13 (26.0) | 5 (10.0) | 18 (18.0) | |
| 3 | 11 (22.0) | 1 (2.0) | 12 (12.0) | |
| Median [IQR] length of surgery, min | 45 [35–65] | 60 [45–80] | 55 [35–70] | 0.005* |
| Intraoperative complications, n (%) | 2 (4.0) | 2 (4.0) | 4 (4.0) | 1.00 |
| Postoperative complications, n (%) | 4 (8.0) | 6 (12.0) | 10 (10.0) | 0.74 |
| Pleural space at 48 h, n (%) | 22 (44.0) | 35 (70.0) | 57 (57.0) | 0.009 |
| Median [IQR] postoperative-LOS, days, n (%) | 4 [3–5] | 5 [4–6] | 5 [4–6] | <0.0001* |
| Median [IQR] total LOS | 6.5 [5–10] | 8 [6–10] | 7.5 [5–10] | 0.09 |
| AL, n (%) | 2 (4.0) | 7 (14.0) | 9 (9.0) | 0.16 |
| Median [IQR] CT duration, days | 3 [2–4] | 4 [3–5] | 4 [3–5] | 0.0001* |
| Prolonged AL, n (%) | 0 (0.0) | 1 (2.0) | 1 (1.0) | 1.00 |
| Median [IQR] POD 1, mL (n=100) | 230 [160–370] | 90 [65–120] | 130 [90–230] | <0.0001* |
| Median [IQR] POD 2, mL (n=100) | 125 [65–200] | 50 [30–80] | 70 [40.0–137.5] | <0.0001* |
| Median [IQR] POD 3, mL (n=83) | 60 [35–120] | 30 [20–60] | 40 [20–70] | 0.004* |
| Median [IQR] POD 4, mL (n=52) | 20 [0–50] | 20 [10–40] | 20 [2.5–40.0] | 0.57 |
| Median [IQR] POD 5, mL (n=26) | 40 [0–50] | 10 [0–50] | 10 [0–50] | 0.36 |
| Median [IQR] POD 6, mL (n=11) | 0 [0–40] | 15 [0–30] | 0 [0–40] | 0.96 |
| Median [IQR] total pleural effusion, mL | 485 [310–700] | 205 [145–260] | 270 [195–490] | <0.0001* |
| Smoke habit, n (%) | ||||
| No smoker | 24 (48.0) | 27 (54.0) | 51 (51.0) | 0.28 |
| Smoker | 23 (46.0) | 23 (46.0) | 46 (46.0) | |
| Former | 3 (6.0) | 0 (0.0) | 3 (3.0) | |
| Median [IQR] VAS POD 1 | 1 [1–1] | 1 [1–2] | 1 [1–1] | 0.08 |
| Median [IQR] VAS POD 2 | 1 [1–1] | 1 [1–2] | 1 [1–1] | 0.03* |
| Median [IQR] VAS POD 3 | 1 [0–1] | 1 [1–2] | 1 [1–1] | 0.21 |
| Median [IQR] VAS POD 4 | 0 [0–1] | 0 [0–0] | 0 [0–1] | 0.39 |
| Loco-regional anaesthesia (n=81), n (%) | 0 (0.0) | 17 (37.8) | 17 (21.0) | <0.0001* |
| Elastomeric pump (n=81), n (%) | 10 (27.8) | 43 (95.6) | 53 (65.4) | <0.0001* |
| NSAID (n=81), n (%) | 36 (100.0) | 28 (62.2) | 64 (79.0) | <0.0001* |
| Morphine (n=81), n (%) | 12 (33.0) | 28 (62.2) | 40 (49.4) | 0.01 |
| 1st month recurrence, n (%) | 0 (0.0) | 1 (2.0) | 1 (1.0) | 1.00 |
| 3rd month recurrence, n (%) | 0 (0.0) | 1 (2.0) | 1 (1.0) | 1.00 |
| 6th month recurrence, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| 1-year recurrence, n (%) | 3 (6.0) | 1 (2.0) | 4 (4.0) | 0.62 |
| Total recurrence, n (%) | 3 (6.0) | 3 (6.0) | 6 (6.0) | 1.00 |
*, Statistically significant. IQR, interquartile ranges; PSP, primary spontaneous pneumothorax; PNX, pneumothorax; UL, upper lobe; LL, lower lobe; ML, middle lobe; CT, computed tomography; LOS, length of stay; AL, air leaks; POD, postoperative day; VAS, visual analogue scale of pain; NSAID, non-steroidal anti-inflammatory drugs.
Considering the small cohort and the age-oriented selection of the population resulting in the absence of statistically significant differences in demographic and clinical data between the TP and IP groups, no propensity score matching analysis was performed.
Surgical indications were a second episode of ipsilateral pneumothorax (88%) and a first contralateral pneumothorax (12%). An active AL, evaluated with a bubbling sign from the chest drainage collection system placed due to respiratory impairment, was recorded in 68% of patients at the time of surgery.
The patients of this study showed lung bullae or parenchymal dysplasia that required intraoperative blebs resection, mainly located in the apex of the upper lobes (84%).
VATS was the standard technique for all procedures: the uniportal approach was used in 70% of patients, showing a statistically significant difference compared to the bi-portal and the tri-portal technique. This difference was more representative between the TP and IP groups, with a statistically significant uniportal approach in the group treated with TP pleurodesis (P<0.0001).
The duration of surgery was significantly lower in the IP group, with a median value of 45 minutes (P=0.005).
Intraoperative complications, such as minor bleeding promptly managed intraoperatively, occurred in 4 patients equally distributed between the two groups. According to the Clavien-Dindo classification, eight patients developed fever related to the pleural sclerosing process (grade I) in the postoperative period. In contrast, pAL and radiological evidence of loculated pleural effusion were observed in 2 more different cases (grade II).
The finding of pleural space, intended as the presence of pleural air in a CR image without clinical sign of AL 48 hours after surgery, was reported in 57% of patients and more represented in patients treated with TP (P=0.009). In addition, either postoperative AL or pAL was recorded sporadically, without a statistically significant difference between the two groups.
The ChT serous output exceeded in terms of total mL collection when IP was used (P<0.0001), with a sensible difference in POD 1 and POD 2 (230 vs. 90 mL and 125 vs. 50 mL, respectively).
The postoperative pain feedback showed equivalent results regarding VAS scores between the two groups. Therefore, the usage of different analgesia methods determined the strongest association of locoregional anesthesia and EIVP with morphine in the TP group (P<0.0001 and P=0.01). On the contrary, venous NSAID was administered more frequently in the IP group (P<0.0001).
The ChT duration was significantly longer in patients treated with TP, and the same result occurred considering the pLOS (P<0.0001).
One-year pneumothorax recurrence was recorded in a total of 6 patients, without a statistically significant difference between IP and TP sclerosing treatments.
A logistic regression analysis to assess the relationship between patients’ demographic and clinical characteristics with the presence of pleural space 48 hours after surgery (Table 2) and total pleural effusion ≥270 mL (Table 3) was performed.
Table 2
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, years | 1.0 (0.9–1.0) | 0.99 | 1.0 (0.9–1.1) | 0.98 | |
| Female | 0.9 (0.4–2.4) | 0.91 | 0.5 (0.1–1.8) | 0.28 | |
| Left side | 1.0 (0.4–2.2) | 0.97 | – | – | |
| PSP-recurrence | 1.0 (0.3–3.6) | 0.92 | – | – | |
| Talc poudrage treatment | 3.0 (1.3–6.8) | 0.01 | 1.5 (0.5–4.1) | 0.47 | |
| Active PNX | 1.5 (0.7–3.5) | 0.33 | – | – | |
| Adherence | 0.9 (0.4–2.1) | 0.88 | – | – | |
| Ports | |||||
| 1 | Reference | Reference | – | – | |
| 2 | 0.2 (0.1–0.6) | 0.004 | 0.4 (0.1–1.4) | 0.14 | |
| 3 | 0.4 (0.1–1.2) | 0.10 | 0.7 (0.2–3.2) | 0.64 | |
| Length of surgery, min | 1.0 (1.0–1.0) | 0.70 | – | – | |
| Intraoperative complications | 0.8 (0.1–5.5) | 0.77 | – | – | |
| Postoperative complications | 7.9 (1.0–64.8) | 0.06 | – | – | |
| Postoperative-LOS, days | 2.0 (1.4–2.9) | <0.0001 | 1.8 (1.0–3.2) | 0.04* | |
| Total LOS | 1.2 (1.0–1.3) | 0.04 | 1.0 (0.9–1.2) | 0.54 | |
| AL | 2.9 (0.6–14.6) | 0.20 | – | – | |
| CT duration, days | 1.9 (1.3–2.8) | 0.0001 | 1.0 (0.5–1.9) | 0.99 | |
| POD 1, mL | 1.0 (1.0–1.0) | 0.18 | – | – | |
| POD 2, mL | 1.0 (1.0–1.0) | 0.81 | – | – | |
| Total pleural effusion, mL | 1.0 (1.0–1.0) | 0.71 | – | – | |
| Former/current smoker | 0.6 (0.3–1.4) | 0.24 | – | – | |
| VAS POD 1 | 1.5 (0.8–2.7) | 0.18 | – | – | |
| VAS POD 2 | 2.1 (1.0–4.7) | 0.05 | – | – | |
| VAS POD 3 | 0.8 (0.4–1.6) | 0.48 | – | – | |
| Loco-regional anaesthesia (n=81) | 1.1 (0.4–3.3) | 0.85 | – | – | |
| Elastomeric pump (n=81) | 2.4 (0.9–6.1) | 0.07 | – | – | |
| NSAID (n=81) | 0.9 (0.3–2.7) | 0.85 | – | – | |
| Morphine (n=81) | 1.6 (0.7–3.9) | 0.31 | – | – | |
*, Statistically significant. PSP, primary spontaneous pneumothorax; PNX, pneumothorax; LOS, length of stay; AL, air leaks; CT, computed tomography; POD, postoperative day; VAS, visual analogue scale of pain; NSAID, non-steroidal anti-inflammatory drugs.
Table 3
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Age, years | 1.0 (0.9–1.1) | 0.87 | 1.0 (0.9–1.1) | 0.32 | |
| Female | 1.3 (0.5–3.3) | 0.56 | 5.6 (1.0–31.5) | 0.05 | |
| Left side | 0.9 (0.4–2.1) | 0.86 | – | – | |
| PSP-recurrence type | 0.4 (0.1–1.6) | 0.20 | – | – | |
| Talc poudrage treatment | 0.07 (0.03–0.19) | <0.0001 | 0.05 (0.01–0.32) | 0.001* | |
| Active PNX | 0.5 (0.2–1.9) | 0.12 | – | – | |
| Adherence | 0.8 (0.4–1.9) | 0.62 | – | – | |
| Ports | |||||
| 1 | Reference | Reference | – | – | |
| 2 | 3.1 (1.0–9.6) | 0.05 | – | – | |
| 3 | 1.2 (0.4–4.0) | 0.78 | – | – | |
| Length of surgery, min | 1.0 (1.0–1.0) | 0.13 | – | – | |
| Intraoperative complications | 1.0 (0.1–7.1) | 0.97 | – | – | |
| Postoperative complications | 1.0 (0.3–3.5) | 0.95 | – | – | |
| Postoperative-LOS, days | 0.9 (0.7–1.1) | 0.14 | – | – | |
| Total LOS | 1.0 (0.9–1.1) | 0.32 | – | – | |
| AL | 0.8 (0.2–3.0) | 0.68 | – | – | |
| CT duration, days | 0.9 (0.7–1.2) | 0.34 | – | – | |
| Former/current smoker | 0.6 (0.3–1.4) | 0.23 | – | – | |
| VAS POD 1 | 0.7 (0.4–1.3) | 0.25 | – | – | |
| VAS POD 2 | 0.7 (0.4–1.3) | 0.27 | – | – | |
| VAS POD 3 | 0.8 (0.4–1.7) | 0.50 | – | – | |
| Loco-regional anaesthesia (n=81) | 0.2 (0.0–0.6) | 0.007 | 0.7 (0.1–3.3) | 0.62 | |
| Elastomeric pump (n=81) | 0.14 (0.05–0.41) | <0.0001 | 0.7 (0.1–4.5) | 0.73 | |
| NSAID (n=81) | 6.4 (1.7–24.5) | 0.007 | – | – | |
| Morphine (n=81) | 0.5 (0.2–1.2) | 0.10 | – | – | |
*, Statistically significant. PSP, primary spontaneous pneumothorax; PNX, pneumothorax; LOS, length of stay; AL, air leaks; CT, computed tomography; POD, postoperative day; VAS, visual analogue scale of pain; NSAID, non-steroidal anti-inflammatory drugs.
Discussion
This observational retrospective study showed that additional pleural painting with iodopovidone was associated with a higher amount of postoperative pleural effusion than talc poudrage pleurodesis. Conversely, shorter drain duration and in-hospital stay were achieved with comparable complications and recurrence rates after surgery. No patients developed severe complications such as acute respiratory distress syndrome (ARDS), confirming the safety of both agents. The only complications recorded within the postoperative days were fever, persistent air leak, and loculated pleural effusion.
Fever was one of the most common complications, and it must be regarded as a response to the induced inflammation of pleura from chemical sclerosing. Other studies showed that talc pleurodesis yielded up to 31% of fever complications (20), while iodopovidone was associated with fever in 6.1–33% of cases (15,16). Another study comparing talc poudrage with iodopovidone solution by tube thoracostomy showed similar frequencies in terms of fever rates in both groups (21). In our study, fever occurred in 8% of both groups and spontaneously recovered by conservative treatment in all patients.
Persistent air leaks showed a low incidence in our study (2% in TP group), similar to other studies reports (22,23), and resolved spontaneously without operative intervention.
Although no statistical difference was found between the recurrence rate in the two groups, some significant differences were shown in the postoperative course.
The presence of a pleural space exhibited by a routine CR 48 hours after surgery was data complementary to the pleural effusion rate from the chest tube. In comparison, the total amount of postoperative pleural effusion was considerably higher in the iodopovidone group, while the incidence of a residual pleural space early after surgery was more frequent in the patients treated with talc poudrage addiction. These data could be motivated by the different pathophysiological pleural effects of the two sclerosing agents. The irritative process seemed to be highly associated with increased exudative production in iodopovidone pleurodesis. On the other hand, talc seemed to give the pulmonary visceral pleura an inelastic effect, reducing its reactive effusion. Moreover, there was a shorter duration of ChT in patients who underwent pleurodesis with iodopovidone (3 vs. 4 days). Based on our experience, unsatisfactory post-surgical CR images motivated the longer duration of drainage when talc poudrage was performed. Multivariate analysis partially explained these findings, as shown in Tables 2,3. Our policy of the timing of tube removal was uniformly applied in both groups. In this regard, the differences in the postoperative course between the two agents were not presupposed, and the difference between the talc- and iodopovidone-induced amounts of drainage and lung healing time was unknown.
These results indicate that iodopovidone may provide a better postoperative course than talc. The mechanism that creates different amounts of drainage remains unclear. Iodopovidone has a low pH and oxidative and cytotoxic properties, which may induce a vigorous inflammatory response and pleural adhesion (21). Furthermore, in our series, the irritative effect could be reasonably magnified by the gentle mechanical abrasion of the parietal pleura done with the soaked sponge technique.
Talc is associated with a high mesothelial response confirmed by an elevation of interleukin 1β (IL-1β) concentration in the culture supernatant (24). Although its efficacy in achieving symphysis of the two pleural layers with a low recurrence rate of PNX is well known (25), its use is still an object of debate because of various complications such as ARDS, chronic pain, and talcomas (26). The talcoma is related to a non-homogeneous laminar distribution of talc over the pleural surface and its aggregation in spot areas. It may represent a confusing factor when CT scans and PET forthcoming investigations are needed, raising suspicions about pleural diseases (27).
This work is a retrospective analysis of outcomes from a relatively small number of patients, and its results should be handled with caution. Thus, due to its retrospective design and the relatively short duration of follow-up, the recurrence rate was possibly underestimated. The distribution of patients who underwent talc or iodopovidone pleurodesis was not uniform over the examination time (as shown in Table S1), representing a potential selection bias. Moreover, the protocol for pain management was not uniform during the study period; therefore, direct pain comparison between the two agents was not possible. Finally, pre- and postoperative pulmonary function tests could not have been performed because most subjects were admitted for respiratory failure and active pneumothorax.
This study confirmed the safety of both talc and iodopovidone for additional pleurodesis. However, in our experience, iodopovidone should be considered over talc poudrage pleurodesis because being associated with a better postoperative recovery. A robust prospective randomized trial would be beneficial to define the advantages of different sclerosing agents for pleurodesis in the surgical management of PSP.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-22-14/rc
Data Sharing Statement: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-14/dss
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-14/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-14/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Cagliari (No. PG/2018/17166). Informed written consent was obtained from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mendogni P, Vannucci J, Ghisalberti M, et al. Epidemiology and management of primary spontaneous pneumothorax: a systematic review. Interact Cardiovasc Thorac Surg 2020;30:337-45. [Crossref] [PubMed]
- Ciriaco P. Special Issue on "Clinical Research of Spontaneous Pneumothorax". J Clin Med 2022;11:2988. [Crossref] [PubMed]
- Noppen M. Spontaneous pneumothorax: epidemiology, pathophysiology and cause. Eur Respir Rev 2010;19:217-9. [Crossref] [PubMed]
- Ayed AK, Chandrasekaran C, Sukumar M. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: clinicopathological correlation. Eur J Cardiothorac Surg 2006;29:221-5. [Crossref] [PubMed]
- How CH, Hsu HH, Chen JS. Chemical pleurodesis for spontaneous pneumothorax. J Formos Med Assoc 2013;112:749-55. [Crossref] [PubMed]
- Tran Z, Haro G, Ebrahimian S, et al. Association of initial management on readmissions for spontaneous pneumothorax in adolescents. Surgery 2022;172:385-90. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Saini N, Nar A, Jabbal HS, et al. Video-Assisted Thoracoscopic Surgery (VATS) for Spontaneous Pneumothorax and Emphysematous Bullous Lung Disease: A Study From Northern India. Cureus 2022;14:e25769. [Crossref] [PubMed]
- Vohra HA, Adamson L, Weeden DF. Does video-assisted thoracoscopic pleurectomy result in better outcomes than open pleurectomy for primary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg 2008;7:673-7. [Crossref] [PubMed]
- Sudduth CL, Shinnick JK, Geng Z, et al. Optimal surgical technique in spontaneous pneumothorax: a systematic review and meta-analysis. J Surg Res 2017;210:32-46. [Crossref] [PubMed]
- Mohamed KH, Hassan OA. A new look at an old agent for pleurodesis. Egypt J Chest Dis Tuberc 2013;62:617-20. [Crossref]
- Cardillo G, Carleo F, Giunti R, et al. Videothoracoscopic talc poudrage in primary spontaneous pneumothorax: a single-institution experience in 861 cases. J Thorac Cardiovasc Surg 2006;131:322-8. [Crossref] [PubMed]
- Dubois L, Malthaner RA. Video-assisted thoracoscopic bullectomy and talc poudrage for spontaneous pneumothoraces: effect on short-term lung function. J Thorac Cardiovasc Surg 2010;140:1272-5. [Crossref] [PubMed]
- Lee YC, Baumann MH, Maskell NA, et al. Pleurodesis practice for malignant pleural effusions in five English-speaking countries: survey of pulmonologists. Chest 2003;124:2229-38. [Crossref] [PubMed]
- Chaari Z, Hentati A, Ben Ayed A, et al. Effectiveness and safety of povidone iodine for prolonged lung air-leak after lung surgery. Asian Cardiovasc Thorac Ann 2022;30:314-20. [Crossref] [PubMed]
- Agarwal R, Khan A, Aggarwal AN, et al. Efficacy & safety of iodopovidone pleurodesis: a systematic review & meta-analysis. Indian J Med Res 2012;135:297-304. [PubMed]
- Tschopp JM, Bintcliffe O, Astoul P, et al. ERS task force statement: diagnosis and treatment of primary spontaneous pneumothorax. Eur Respir J 2015;46:321-35. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Gift AG. Visual analogue scales: measurement of subjective phenomena. Nurs Res 1989;38:286-8. [Crossref] [PubMed]
- Park EH, Kim JH, Yee J, et al. Comparisons of doxycycline solution with talc slurry for chemical pleurodesis and risk factors for recurrence in South Korean patients with spontaneous pneumothorax. Eur J Hosp Pharm 2019;26:275-9. [Crossref] [PubMed]
- Agarwal R, Paul AS, Aggarwal AN, et al. A randomized controlled trial of the efficacy of cosmetic talc compared with iodopovidone for chemical pleurodesis. Respirology 2011;16:1064-9. [Crossref] [PubMed]
- Cardillo G, Bintcliffe OJ, Carleo F, et al. Primary spontaneous pneumothorax: a cohort study of VATS with talc poudrage. Thorax 2016;71:847-53. [Crossref] [PubMed]
- Horio H, Nomori H, Kobayashi R, et al. Impact of additional pleurodesis in video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax. Surg Endosc 2002;16:630-4. [Crossref] [PubMed]
- Mierzejewski M, Paplinska-Goryca M, Korczynski P, et al. Primary human mesothelial cell culture in the evaluation of the inflammatory response to different sclerosing agents used for pleurodesis. Physiol Rep 2021;9:e14846. [Crossref] [PubMed]
- Hunt I, Barber B, Southon R, et al. Is talc pleurodesis safe for young patients following primary spontaneous pneumothorax?. Interact Cardiovasc Thorac Surg 2007;6:117-20. [Crossref] [PubMed]
- Milton R, Cale AR. Chronic pain due to talc pleurodesis for spontaneous pneumothorax. Ann Thorac Surg 2003;76:1740-1. [Crossref] [PubMed]
- Kwek BH, Aquino SL, Fischman AJ. Fluorodeoxyglucose positron emission tomography and CT after talc pleurodesis. Chest 2004;125:2356-60. [Crossref] [PubMed]
Cite this article as: Ferrari PA, Santoru M, Pinna-Susnik M, Grimaldi G, Murenu A, Riva L, Sarais S, Sotgiu G, Tamburrini A, Cherchi R. Additional pleural painting with iodopovidone during video-assisted thoracoscopic bullectomy for primary spontaneous pneumothorax: an observational, single-center retrospective analysis. Shanghai Chest 2022;6:20.


