
Surgical management of parapneumonic empyema
Introduction
Empyema is a complex entity with multifactorial pathogenesis and aetiology. It involves the accumulation of pus in the pleural space, and it is considered an advanced stage of the initial parapneumonic effusion (PPE) in which the fluid is usually a mixture of proteins, leukocytes (particularly neutrophils), cellular debris, and bacteria. These effusions are mainly secondary to pneumonia. There are other less common aetiologies for pleural empyema including infected hemothorax, ruptured lung abscess, oesophageal tears, and thoracic trauma (1,2). Rapid diagnosis is essential to successful treatment and patient survival.
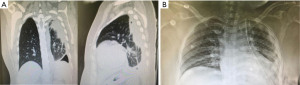
Classically, patients with empyema present with fever, chills, cough, or chest discomfort, and they might succumb to worsening sepsis, septic shock, or death if it is not rapidly or adequately managed (3). Chest X-ray might show an opacity with a meniscus sign when the viscous fluid sticks to the parietal pleura, or lenticular shape if the fluid has a convex shape or diffuse opacification of the pleural cavity which is usually seen in large empyema. Ultrasound usually reveals a fluid collection. Chest computed tomography (CT) scan also shows thickening of the pleural membranes due to fibrin adherence causing vascular proliferation, known as the split pleura sign (4); furthermore, the CT can show septations in stage 2 of the disease.
A diagnosis is made by pleural fluid analysis and culture through thoracocentesis to identify the causative agent (5). The aspirate might show purulent fluid or turbid fluid. Pleural fluid with a pH below 7.20 or a glucose level below 60 mg/dL or a lactate dehydrogenase level of more than three times the upper normal limit for serum is a positive indication of parapneumonic empyema (6).
The stages of parapneumonic empyema can be classified as uncomplicated effusion, complicated effusion, and pleural empyema. The stages of empyema vary according to the composition of the exudate. Stage 1 (exudative) involves the accumulation of pus or infected fluid; stage 2 (fibrinopurulent) starts when fibrin infiltrates and forms different septations and pockets in the pleural cavity, and finally, stage 3 (organizing) begins when a thick restrictive fibrous cover is formed around the lung, trapping it (2,7).
The management of empyema can be challenging and complex. The early involvement of a multidisciplinary team that includes thoracic surgeons, chest physicians, infectious disease specialists, experienced nursing staff, and respiratory therapists is prudent in improving morbidity and mortality. In fact, the American Association for Thoracic Surgery (ATS), British Thoracic Society (BTS) and American College of Chest Physicians (ACCP) have published comprehensive, evidence-based guidelines to help guide healthcare professionals in their treatment of empyema. These highly skilled physicians can help identify surgical candidates early, assess thoracic surgical risk and manage potential complications associated with invasive procedures (3).
The goal is to combine medical and surgical treatment to target the source of the infection and ensure adequate lung re-expansion since acute empyema can have long term consequences despite adequate therapeutic interventions, such as pleural fibrosis that can lead to adhesions, decreased lung compliance, and a restrictive lung disease pattern (3,8).
The initial medical approach will depend on whether the bacterial agent is known, the bacterial species, and the stage and severity of the empyema. Medical management is divided into empirical treatment and definitive treatment. Empirical management starts by identifying the source of the infection. Patients with community-acquired agents are started on either a combination therapy of parenteral second- or third-generation cephalosporin with metronidazole or clindamycin for anaerobic coverage (9). The therapy for hospital-acquired infections is used to additionally cover methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (9).
Definitive treatment will depend on the stage of empyema. Stage 1 usually involves the insertion of a chest tube to empty the infected fluid chest tube insertion but when it fails, other strategies are considered, such as fibrinolytic agents that might be considered to help with the drainage. However, studies have not confirmed the benefits of this early stage (7). Stage 2 empyema is usually drained by chest tube placement and then intrapleural fibrinolytic treatment or video-assisted thoracoscopic surgery (VATS) debridement is used if the chest tube is ineffective. Patients in stage 3 have been classically treated by open decortication and pleurectomy through thoracotomy (7); however, VATS decortication is feasible and is becoming more popular among thoracic surgeons.
Finally, empyema carries a poor prognosis if not treated early and aggressively from the time of the diagnosis. Although most patients recover, clinical outcomes remain poor with one in five patients requiring surgery and 20% dying within the first year of diagnosis. Also, there is a 1.5-times increase in negative outcomes in the frail, elderly, and immunocompromised population (3).
Herein, we present an exhaustive literature review of the surgical management of parapneumonic empyema, in addition to our experience with the emergent uniportal, and a novel perimammary uniportal VATS decortication introduced by our team for stages 2 and 3 (10).
Intrapleural fibrinolytic treatment
Although it has been used to manage PPE and parapneumonic empyema for the past 70 years, intrapleural fibrinolytic therapy is considered a controversial therapeutic modality with yet insufficient evidence (11). The current American and British guidelines for managing PPE and parapneumonic empyema recommend proceeding to surgical management if chest tube drainage is ineffective, with avoiding the routine use of fibrinolytic therapy (12-14). Fibrinolytic agents, such as streptokinase, urokinase, tissue plasminogen activator (tPA), and recombinant tissue plasminogen activator (rtPA) work by converting plasminogen into plasmin, a fibrinolytic agent. Thus, degrading fibrin and causing deposition of loculations and septations that start forming in the fibrinopurulent stage of empyema. Two deoxyribonucleases (DNase) have been used in pleural infection as well; Dornase alfa is a recombinant DNase that works as a mucolytic (15,16).
Several methods are used to manage empyema with the agents mentioned above; however, the literature has shown that using fibrinolytic agents on their own does not manage empyema adequately, and it is no longer a viable option. The most positive results were achieved by administering tPA and DNase together. The Multicenter Intrapleural Sepsis Trials (MIST) 1 and 2 studied the effect of fibrinolytic agents and DNase administration on different parameters. MIST1 concluded that streptokinase increased pleural fluid drainage, but it did not have prominent effects on the patient’s overall recovery (17). MIST2 showed that the combination of intrapleural tPA, and DNase improved the pleural drainage and reduced the hospital length of stay. In addition, there was a 75% reduction in the need for surgical interventions (18). MIST2 is the only clinical trial that tested the treatment. It also showed the usage of fibrinolytic therapy alone does not effectively treat empyema.
The combination therapy of fibrinolytic agents and DNase might be the only option for patients who are not surgical candidates (19). The most threatening complication of fibrinolytic agents’ administration is a hemothorax since it can cause more lung entrapment, and it is usually an indication for VATS. The risk is higher for patients with coagulopathies and renal failure (20). Other complications include chest pain, fever, and allergic reactions that present more with streptokinase (21).
The usage of fibrinolytic therapy for empyema requires further investigation and understanding of the mechanism, dosing, timing of usage, and adverse effects (11).
Preoperative assessment
General preoperative assessment for empyema starts with obtaining surgical consent after taking a thorough medical history, doing a physical examination, and performing multiple laboratory and imaging investigations. In addition to pleural fluid analysis, laboratory tests include analysing a complete blood count with differential, basic metabolic panel, and a coagulation profile (22). Imaging studies start with a conventional chest X-ray, with a lateral decubitus view being the most sensitive (23). A chest X-ray is the most used as an imaging study due to its availability and accessibility; however, complex cases of PPE might be missed or misevaluated doing an X-ray alone (24). Therefore, a chest X-ray is often combined with contrast-enhanced CT. CT allows for a more detailed visualization of the chest, and it might reveal the underlying pathology causing the PPE (25-27).
Preoperative assessment is imperative for the patient’s risk stratification and perioperative management. It guides the healthcare team, especially the surgeon, through the decision-making process. Preoperative management includes evaluating the candidate’s ability to undergo surgery in the first place, adjusting the timing of the surgery accordingly, and performing preoperative supportive measures, such as chest physiotherapy, when necessary. Based on the data, implementing such strategies prevents and minimizes the intra and postoperative unfortunate events, reduces complications rate, as well as morbidity and mortality, decreases the hospital length of stay, and improves the resources used and the patient’s overall experience and recovery (28). Major complications in thoracic surgery include atelectasis, persistent air leak, pneumonia, and respiratory failure. They occur in 15–20% of patients and account for most of the 3–4% of mortality rate (6).
Surgical approaches and techniques
Early evaluation and intervention contribute greatly to the outcome of patients with empyema (29). Surgical management is mainly indicated in stage 2 empyema and above when other non-surgical modalities do not adequately manage and treat the condition. Non-surgical management includes antibiotic therapy, intrapleural fibrinolytic therapy, and chest tube drainage. When such measures fail, surgery is indicated usually in advanced stages. The goal of the intervention is to achieve complete pleural fluid evacuation and full lung expansion. This is done by tissue debridement or pleural decortication that can be surgically achieved through either VATS or open thoracotomy (30).
Open decortication has been the gold standard for the surgical management of empyema, especially in advanced stages, stage 2 and chronic empyema. However, the now evolving VATS is often considered a better alternative with comparable outcomes even in managing advanced stages (31).
Decortication is based on the removal of the fibrous peel overlying the surface of the lung along with the granulation tissue to allow lung re-expansion. Open decortication is done under general anaesthesia with single lung ventilation and the patient is in a lateral decubitus position. The pleural cavity is entered through the lateral or posterolateral thoracotomy at the level of the fifth or sixth intercostal space. After the pleural abscess cavity is fully excised, the junction of the visceral cortex is entered by sharp or blunt dissection until the lung parenchyma is identified and monitored to re-expand. After mobilizing the lung and freeing it along the chest wall and diaphragm, with extra caution around the apices, careful dissection of the visceral pleura is performed. The success of the decortication depends on the lung’s elasticity to fill the cavity after freeing it. Applying some positive pressure during the decortication might help by providing a counter-pressure during dissection but may increase the incidence of postoperative air leak. In fact, persistent air leak is a common complication; thus, pneumostasis and haemostasis must be achieved to minimize it (32).
Despite many surgeons being used to performing open thoracotomies in deference to VATS due to the new exposure and lack of expertise, VATS has become the superior approach and the usual first-line surgical intervention for stage 2 and even stage 3 of PPE in experienced hands. VATS decortication is performed under general anaesthesia with single lung ventilation and the patient is in a lateral decubitus position as well.
Initially, multiportal VATS has been the minimally invasive approach of choice to deal with empyema. It provides the advantage to allow entering the thoracic cavity from different angles.
Classically, a 2-cm incision is then created inferior and anterior to the inferior angle of the scapula for the thoracoscope to enter. Another way is to insert the first port in the fourth intercostal space in the anterior axillary line and the other ports in the sixth or seventh intercostal space. Initially, the pleural debridement is performed using directed suction with a sucker or a modified 36F catheter gauge intercostal tube combined with saline lavage. The lung is mobilized and separated from the parietal pleura along the chest wall and diaphragm, with extra caution around the apices, and then the visceral pleura is dissected carefully. The ability of the lung to re-expand is now monitored. A positive pressure might be applied to the operated lung to facilitate the decortication. More visceral decortication might be performed if the lung fails to re-expand. After decortication, the pleural cavity is usually irrigated with saline, with or without aqueous betadine. Hemostasis must be ensured to avoid air leaks and other common complications.
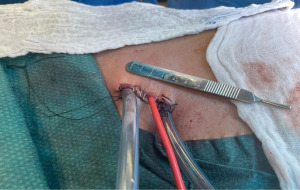
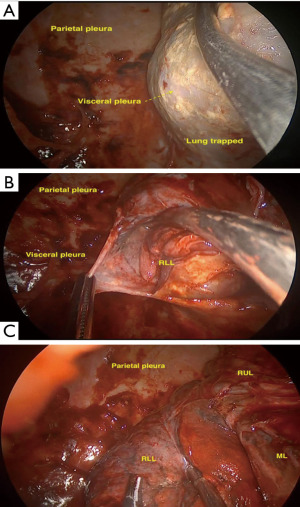
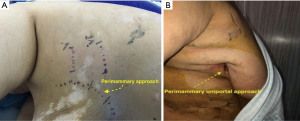
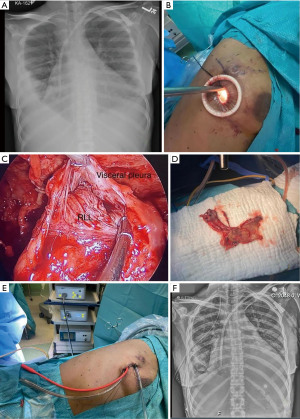
After the introduction of uniportal VATS (33,34), some experienced surgeons have adopted it to perform uniportal VATS decortication even in advanced stages (Figure 1). VATS has proven to be easier to perform and manage with more positive outcomes compared to open thoracotomies (12). VATS is associated with better postoperative outcomes including a less hospital length of stay, less postoperative pain and complications rate and more appealing cosmetic results (Figure 2). VATS is also associated with less 30-day mortality. This is also attributed to a better immune function post VATS when compared to thoracotomy since it has been shown that circulating immune cells are higher in activity and efficiency post VATS than after thoracotomy (35).
The process of lung mobilization from the chest wall and decortication usually tend to ooze or bleed. This contributes to the formation and presence of clots and haemorrhagic effusion during the surgery. Intrapleural fibrinolysis can be activated during surgery, which makes it more difficult to control the bleeding intraoperatively and might lead to postoperative bleeding. The use of some agents with certain hemostatic properties, such as hydrogen peroxide or tranexamic acid might reduce the intrapleural fibrinolysis and the risk of postsurgical hemothorax, or even contribute to the sterilization of the pleural cavity (36). Moreover, when the bleeding from the chest wall is still significant, intraoperative packing can be applied in combination with local hemostatic agents and blood products transfusion to stop the active bleeding (37).
Another way to reduce both the risk of clots and the activation of the intrapleural fibrinolysis is the use of continuous irrigation of the pleural cavity after surgery. This decreases the risk of postoperative hemothorax and reoperation. It can be done by using a rubber catheter inserted into the single port of the uniportal approach or one of the ports in multiportal VATS (38).
Parietal pleurectomy during decortication has been described as one of the cornerstones of the surgical management of the empyema, especially for patients who are unfit for radical procedures (32). It improves the pulmonary function and chest wall diameters (39); however, it is not usually performed through VATS which may significantly reduce the risk of postoperative bleeding and surgery time overall in risky patients with comorbidities and potential risk of postoperative bleeding (32). We have learnt from our own experience that the functionality of the chest wall is not affected, and the parietal pleura becomes normal in the long-term image workup.
Postoperative air leak is one of the expected and common complications after decortication. There is some evidence on how VATS reduces the presence and intensity of postoperative air leak since the decortication is more precise and less deep than the one achieved by thoracotomy. Moreover, performing decortication through uniportal VATS, although requiring experience in minimally invasive surgery, might reduce the presence of prolonged air leak due to better exposure and magnification of the surgical field, especially, if it is done using the anterior to posterior lung approach—from lees adherent areas of the visceral pleura to the thickest ones (32).
Finally, in the case of persistent empyema that is refractory to standard therapeutic modalities that include surgery, creating an open thoracostomy window could be considered to apply. It might be a permanent drainage solution, or it might be closed later with a muscle flap or omental pedicle. An open thoracostomy window allows for dependent drainage and frequent packing (3,12).
Postoperative management
The early postoperative outcome is crucial, and patients should be monitored carefully. ICU admission is advised if the patient has any comorbidities, such as a reduced cardiopulmonary reserve, life-threatening organ failure, and renal or liver failure.
Intensive cardiorespiratory monitoring, proper management of thoracic drainages, aggressive pain control (multimodal analgesia and regional anaesthetic techniques), deep vein thrombosis (DVT) prophylaxis, and multimodal rehabilitation are important to avoid any complications within the post-surgical period (12).
Post-surgical regional anaesthetic techniques have been shown to decrease the likelihood of future chronic pain in patients who underwent thoracotomy (40,41). The technique includes different fascial plane chest wall blocks depending on the surgical site. Upper anterolateral wall analgesia is accomplished by blocking either medial and lateral pectoral nerves or lateral cutaneous branches of intercostal nerves, approximately T2–T6. Furthermore, an erector spinae plane block inhibits the spinal nerve’s dorsal and ventral rami resulting in anterior, lateral, and posterior chest wall analgesia (42). On the other hand, the gold standard pain control analgesia for open thoracotomies includes thoracic epidural analgesia (TEA), and paravertebral block (PVB) (43). Studies have shown that paravertebral block is associated with fewer side effects than epidural analgesia (44).
Early mobilization and exercise are recommended to promote lung expansion, decrease venous stasis, and prevent deep vein thrombosis/pulmonary embolism (35). Further postoperative complications can include hemothorax, prolonged air leakage, respiratory failure, arrhythmias, respiratory infections, atelectasis, and thromboembolic lung disease.
Discussion
Approximately 5–10% of patients with PPE develop empyema eventually (45). Early detection and management of PPE, and subsequently empyema, is vital for limiting the disease and reducing morbidity and mortality (46). Management includes antibiotic therapy, intrapleural fibrinolytic therapy, chest tube insertion, and surgical management either through VATS or thoracotomy. The end goal of the surgical management of empyema is to achieve full evacuation of pleural exudates, and full lung expansion (47).
Open decortication continues to be the gold standard in advanced stages of empyema; however, VATS decortication has been gaining popularity over the last decade, and it is currently extensively used in managing empyema (48). Mainly based on its technical advantages compared to open surgery, VATS allows for better visualization of the surgical field, better accessibility to pleural collections, and magnification of the visceral pleura that help facilitate the evacuation of the pleural cavity and the decortication.
Postoperatively, VATS reduces the 30-day mortality rate (49,50). It also results in both less ICU and hospital length of stay, in addition to less postoperative pain, fewer complication rates, better cosmetic results and less immunosuppression which might reduce the risk of recurrence and contribute to the patient’s faster recovery (51). Moreover, it has been recently shown that uniportal VATS causes a less inflammatory response and less immunosuppression than multiportal VATS. That might need to be taken into consideration in patients with comorbidities when choosing the surgical approach (33).
One of the challenges facing the surgical management of patients is obesity. It is now considered a state of active low-grade inflammation with several implications (52). Although obesity is an epidemic, and it is related to many contributing factors to morbidity, such as diabetes mellitus, extensive research needs to be done to optimize surgical management in obese patients. Moreover, obesity has been associated with an increased rate of nosocomial and wound infection post-surgery (53). Risk-associated implications of obesity, therefore, affect the perioperative management, including the surgical approach choice.
In the era of advancement of the minimally invasive approach, several studies support the choice of laparoscopy over laparotomy when managing obese patients (54,55). There has not been a study comparing thoracoscopy to thoracotomy in managing empyema in obese patients; nonetheless, a decreased rate of complications is vastly observed in the literature comparing VATS to thoracotomy. An analysis of the Italian VATS group registry concluded that VATS lobectomy could be safely used in morbidly obese patients without compromising the operation and oncological outcome (56). In our institution, we have performed several decortication procedures through VATS on obese patients, and we achieved positive results in all.
Other factors contributing to the choice of performing VATS, thoracotomy, or even converting VATS to thoracotomy are the degree of expertise of the surgeon, the patient’s condition, the complexity of the case, and both the technology and the quality of instruments available in the operating room. Even the decision of performing multiportal or uniportal VATS depends on the complexity of the case as well as the expertise of the surgeon (12). Patients with comorbidities, such as liver or renal failure or cardiomyopathy have been considered a classic indication for open decortication; however, these patients can be safely operated on by VATS, and even uniportal VATS in experienced hands (36,57).
In this sense, uniportal VATS decortication for stage 3 empyema requires strong surgical skills, experience, and adequate technology to be successful, but it is gaining popularity. Nevertheless, the uniportal anterior approach through the fifth or sixth intercostal space has certain advantages compared to the multiportal VATS since it minimizes the risk of lung or diaphragmatic injury while entering the pleural cavity. It also allows for clear visualization of the surgical field and a faster mobilization of the lung, as well as a better exposure of the collections inside the pleural cavity. Lung decortication is facilitated when the lung is approached anteriorly since the pleura is thicker and more adherent to the lung on the posterior aspect (Figure 3, Video 1).
Finally, despite the advances achieved in minimally invasive decortication over the last decades, there is still room for some innovations. In this sense, we have described, for the first time, the perimammary uniportal VATS decortication; a novel approach for female patients (Video 2) which is performed by entering the pleural cavity in the perimammary fold through the fifth or sixth intercostal space. This anterior approach provides a wide visualization of the pleural cavity as well and facilitates lung mobilization and decortication since the lung and the empyema cavity are approached from anterior to posterior. The exposure achieved through the perimammary approach is optimal and reduced instrumental collisions and handling difficulties, allowing us to safely deal with complex chronic empyema (Video 3). Also, it provides patients with low pain, a short length of hospital-stay, faster recovery, and better cosmetic results due to a less-visible scar, eventually (Figures 4-6).
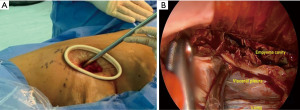
Robotic-assisted thoracoscopic decortication is another promising innovation due to some advantages provided by the robot, such as a 3D magnified vision. This facilitates fine dissection of dense adhesions with minimal blood loss and less pulmonary parenchymal injury in addition to the ability of the robotic EndoWrist to allow maximum and safe manipulation at the thoracic outlet (58,59). However, it is still costly, and surgically challenging due to the lack of pleural space to achieve an adequate range of motion for the robotic arms and the need of approaching the pleural cavity by using multiple ports. Therefore, it is only currently performed in very few centres worldwide.
Further research is warranted to prove the benefits of these novel approaches for minimally invasive lung decortication and to define an optimal surgical management strategy to deal with empyema that improves patient outcomes and reduces morbidity and mortality.
Acknowledgments
We would like to thank Dr. Adil Maqbool, Dr. Hirdah Rana, and Ms. Cristina Riera López for their technical support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Rahul Nayak) for the series “Management of Pleural Diseases in the 21st Century” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-11/coif). The series “Management of Pleural Diseases in the 21st Century” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldman L, Schafer AI. Goldman-Cecil Medicine, 2-Volume Set. Philadelphia, PA: Elsevier; 2019.
- Kasper DL, Fauci AS, Hauser S, et al. Harrisons Principles of Internal Medicine. New York, NY: McGraw-Hill Medical Publishing Division; 2016.
- Iguina MM, Danckers M. Thoracic Empyema. Treasure Island (FL): StatPearls Publishing; July 19, 2021.
- Kraus GJ. The split pleura sign. Radiology 2007;243:297-8. [Crossref] [PubMed]
- Godfrey MS, Bramley KT, Detterbeck F. Medical and Surgical Management of Empyema. Semin Respir Crit Care Med 2019;40:361-74. [Crossref] [PubMed]
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80. [Crossref] [PubMed]
- Ahmed AE, Yacoub TE. Empyema thoracis. Clin Med Insights Circ Respir Pulm Med 2010;4:1-8. [Crossref] [PubMed]
- Reichert M, Hecker M, Witte B, et al. Stage-directed therapy of pleural empyema. Langenbecks Arch Surg 2017;402:15-26. [Crossref] [PubMed]
- Bernstein WK, Deshpande S. Preoperative evaluation for thoracic surgery. Semin Cardiothorac Vasc Anesth 2008;12:109-21. [Crossref] [PubMed]
- Migliore M. Efficacy and safety of single-trocar technique for minimally invasive surgery of the chest in the treatment of noncomplex pleural disease. J Thorac Cardiovasc Surg 2003;126:1618-23. [Crossref] [PubMed]
- Idell S, Rahman NM. Intrapleural Fibrinolytic Therapy for Empyema and Pleural Loculation: Knowns and Unknowns. Ann Am Thorac Soc 2018;15:515-7. [Crossref] [PubMed]
- Shen KR, Bribriesco A, Crabtree T, et al. The American Association for Thoracic Surgery consensus guidelines for the management of empyema. J Thorac Cardiovasc Surg 2017;153:e129-46. [Crossref] [PubMed]
- Davies HE, Davies RJ, Davies CW, et al. Management of pleural infection in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii41-53. [Crossref] [PubMed]
- Tillett WS, Sherry S, Read CT. The use of streptokinase-streptodornase in the treatment of chronic empyema; with an interpretive discussion of enzymatic actions in the field of intrathoracic diseases. J Thorac Surg 1951;21:325-41. [Crossref] [PubMed]
- Piccolo F, Popowicz N, Wong D, et al. Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection. J Thorac Dis 2015;7:999-1008. [PubMed]
- Kwon YS. Pleural infection and empyema. Tuberc Respir Dis (Seoul) 2014;76:160-2. [Crossref] [PubMed]
- Maskell NA, Davies CW, Nunn AJ, et al. U.K. Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med 2005;352:865-74. [Crossref] [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Semenkovich TR, Olsen MA, Puri V, et al. Current State of Empyema Management. Ann Thorac Surg 2018;105:1589-96. [Crossref] [PubMed]
- Gervais DA, Levis DA, Hahn PF, et al. Adjunctive intrapleural tissue plasminogen activator administered via chest tubes placed with imaging guidance: effectiveness and risk for hemorrhage. Radiology 2008;246:956-63. [Crossref] [PubMed]
- Ozcelik C, Inci I, Nizam O, et al. Intrapleural fibrinolytic treatment of multiloculated postpneumonic pediatric empyemas. Ann Thorac Surg 2003;76:1849-53; discussion 1853. [Crossref] [PubMed]
- Chalmers JD, Singanayagam A, Murray MP, et al. Risk factors for complicated parapneumonic effusion and empyema on presentation to hospital with community-acquired pneumonia. Thorax 2009;64:592-7. [Crossref] [PubMed]
- Karkhanis VS, Joshi JM. Pleural effusion: diagnosis, treatment, and management. Open Access Emerg Med 2012;4:31-52. [Crossref] [PubMed]
- Brixey AG, Luo Y, Skouras V, et al. The efficacy of chest radiographs in detecting parapneumonic effusions. Respirology 2011;16:1000-4. [Crossref] [PubMed]
- Rahman NM, Chapman SJ, Davies RJ. Pleural effusion: a structured approach to care. Br Med Bull 2004;72:31-47. [Crossref] [PubMed]
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii4-17. [Crossref] [PubMed]
- Matheos T, Ram L, Canelli R. Preoperative Evaluation for Thoracic Surgery. Thorac Surg Clin 2020;30:241-7. [Crossref] [PubMed]
- Slinger PD, Johnston MR. Preoperative assessment for pulmonary resection. Anesthesiol Clin North Am 2001;19:411-33. [Crossref] [PubMed]
- Bilgin M, Akcali Y, Oguzkaya F. Benefits of early aggressive management of empyema thoracis. ANZ J Surg 2006;76:120-2. [Crossref] [PubMed]
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007;45:1480-6. [Crossref] [PubMed]
- Perikleous P, Rathinam S, Waller DA. VATS and open chest surgery in diagnosis and treatment of benign pleural diseases. J Vis Surg 2017;3:84. [Crossref] [PubMed]
- Rathinam S, Waller DA. Pleurectomy decortication in the treatment of the "trapped lung" in benign and malignant pleural effusions. Thorac Surg Clin 2013;23:51-61. vi. [Crossref] [PubMed]
- Ismail M, Swierzy M, Nachira D, et al. Uniportal video-assisted thoracic surgery for major lung resections: pitfalls, tips and tricks. J Thorac Dis 2017;9:885-97. [Crossref] [PubMed]
- Ismail M, Nachira D, Meacci E, et al. Uniportal video-assisted thoracic surgery in the treatment of pleural empyema. J Thorac Dis 2018;10:S3696-703. [Crossref] [PubMed]
- Ng CS, Wan IY, Yim AP. Impact of video-assisted thoracoscopic major lung resection on immune function. Asian Cardiovasc Thorac Ann 2009;17:426-32. [Crossref] [PubMed]
- Terra RM, Waisberg DR, Almeida JL, et al. Does videothoracoscopy improve clinical outcomes when implemented as part of a pleural empyema treatment algorithm? Clinics (Sao Paulo) 2012;67:557-64. [Crossref] [PubMed]
- Heyns M, Knight P, Steve AK, et al. A Single Preoperative Dose of Tranexamic Acid Reduces Perioperative Blood Loss: A Meta-analysis. Ann Surg 2021;273:75-81. [Crossref] [PubMed]
- Pérez-Alonso D, Santana-Rodríguez N, Cano JR, et al. Selective packing for uncontrollable traumatic thoracic wall bleeding preserving cardiopulmonary function. Am J Surg 2017;214:413-5. [Crossref] [PubMed]
- Hooper CE, Edey AJ, Wallis A, et al. Pleural irrigation trial (PIT): a randomised controlled trial of pleural irrigation with normal saline versus standard care in patients with pleural infection. Eur Respir J 2015;46:456-63. [Crossref] [PubMed]
- Meier AH, Hess CB, Cilley RE. Complications and treatment failures of video-assisted thoracoscopic debridement for pediatric empyema. Pediatr Surg Int 2010;26:367-71. [Crossref] [PubMed]
- Fredheim OM, Borchgrevink PC, Kvarstein G, et al. Post-operative pain management in hospitals. Tidsskr Nor Laegeforen 2011;131:1772-6. [Crossref] [PubMed]
- Gottschalk A, Cohen SP, Yang S, et al. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594-600. [Crossref] [PubMed]
- Kelava M, Alfirevic A, Bustamante S, et al. Regional Anesthesia in Cardiac Surgery: An Overview of Fascial Plane Chest Wall Blocks. Anesth Analg 2020;131:127-35. [Crossref] [PubMed]
- Joshi GP, Bonnet F, Shah R, et al. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg 2008;107:1026-40. [Crossref] [PubMed]
- Morris BA, Benetti M, Marro H, et al. Clinical practice guidelines for early mobilization hours after surgery. Orthop Nurs 2010;29:290-316; quiz 317-8. [Crossref] [PubMed]
- Shebl E, Paul M. Parapneumonic Pleural Effusions And Empyema Thoracis 2022.
- Van Schil PE, Hendriks JM, Lauwers P. Focus on treatment complications and optimal management surgery. Transl Lung Cancer Res 2014;3:181-6. [PubMed]
- Angelillo Mackinlay TA, Lyons GA, Chimondeguy DJ, et al. VATS debridement versus thoracotomy in the treatment of loculated postpneumonia empyema. Ann Thorac Surg 1996;61:1626-30. [Crossref] [PubMed]
- DE LA Torre M. Uniportal VATS lobectomy. Minerva Chir 2016;71:46-60. [PubMed]
- Farjah F, Backhus LM, Varghese TK, et al. Ninety-day costs of video-assisted thoracic surgery versus open lobectomy for lung cancer. Ann Thorac Surg 2014;98:191-6. [Crossref] [PubMed]
- Chambers A, Routledge T, Dunning J, et al. Is video-assisted thoracoscopic surgical decortication superior to open surgery in the management of adults with primary empyema? Interact Cardiovasc Thorac Surg 2010;11:171-7. [Crossref] [PubMed]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860-7. [Crossref] [PubMed]
- Quante M, Dietrich A, ElKhal A, et al. Obesity-related immune responses and their impact on surgical outcomes. Int J Obes (Lond) 2015;39:877-83. [Crossref] [PubMed]
- Lee J, Mabardy A, Kermani R, et al. Laparoscopic vs open ventral hernia repair in the era of obesity. JAMA Surg 2013;148:723-6. [Crossref] [PubMed]
- Vargas GM, Sieloff EP, Parmar AD, et al. Laparoscopy decreases complications for obese patients undergoing elective rectal surgery. Surg Endosc 2016;30:1826-32. [Crossref] [PubMed]
- Guerrera F, Lyberis P, Lausi PO, et al. Does morbid obesity influence perioperative outcomes after video-assisted thoracic surgery (VATS) lobectomy for non-small cell lung cancer? Analysis of the Italian VATS group registry. Surg Endosc 2022;36:3567-73. [Crossref] [PubMed]
- Ye B, Wang M. Video-assisted Thoracoscopic Surgery versus Thoracotomy for Non-Small Cell Lung Cancer: A Meta-Analysis. Comb Chem High Throughput Screen 2019;22:187-93. [Crossref] [PubMed]
- Perikleous P, Rathinam S, Waller DA. VATS and open chest surgery in diagnosis and treatment of benign pleural diseases. J Vis Surg 2017;3:84. [Crossref] [PubMed]
- Khan AZ, Khanna S, Agarwal N, et al. Robotic thoracic surgery in inflammatory and infective diseases. Ann Cardiothorac Surg 2019;8:241-9. [Crossref] [PubMed]
Cite this article as: Santana-Rodríguez N, Aldebakey H, Albalkhi I, Hussein M, Alshammari A, Ahmed A, Alshariff N, Albeyali H, Hashim M, Clavo-Varas B, Migliore M. Surgical management of parapneumonic empyema. Shanghai Chest 2022;6:25.


