Urgent lung transplant in acute pulmonary scleroderma: a case report
Introduction
Systemic scleroderma (SSc) is an acute and chronic autoimmune connective tissue disease (CTD) that may involve the skin, vessels, and internal organs, with a severe progressive fibrotic evolution. It mainly affects the young female population, with Afro-Americans having reported an earlier onset and more severe cases (1). The progression of SSc is still characterized by a significant mortality ratio, ranging from 2.6 to 3.5 (2). The estimated survival is 75% at 5 years and 62.5% at 10 years from diagnosis and pulmonary involvement represents the main cause of death (3,4). Some degree of pulmonary involvement is seen in more than 80% of patients with SSc. The main clinical manifestations are interstitial lung disease (ILD) (also called fibrosing alveolitis or pulmonary fibrosis) and pulmonary vascular disease, leading to pulmonary arterial hypertension (PAH). The incidence of ILD and PAH in patients with SSc pulmonary involvement is 70% and 10–12%, respectively (5).
Literature already supports lung transplant in autoimmune diseases like SSc with a progressive and chronic deterioration of lung parenchyma and respiratory function (6-8). In these cases, patients undergoing bilateral lung transplantation for SSc have a similar 1- and 5-year survival as those with restrictive lung disease (9,10).
Veno-venous extracorporeal membrane oxygenation (V-V ECMO) has been used for years as a bridge to lung transplant in patients with end-stage restrictive lung disease secondary to scleroderma (11,12). However, few case reports describe the use of V-V ECMO in rapidly progressive ILD (RPILD) arising as an acute complication of an autoimmune CTD. Huang et al. reported successful outcomes in a patient with amyopathic dermatomyositis (ADM) complicated by severe acute ILD who received early V-V ECMO therapy in combination with double filtration plasmapheresis (DFPP) (13). DFPP is a semi-selective blood purification technique derived from the plasma exchange modality that can rapidly remove the pathogenetic antibodies and immune complexes efficiently (14). Marchiset et al. and Leclair et al. described the use of rescue V-V ECMO therapy as a bridge to lung transplant in the context of a RPILD secondary to CTD (15,16).
Our paper reports a case of an awake rescue V-V ECMO in SSc with acute onset of lung failure, the therapeutic strategies of bridging to lung transplant, and early outcomes. We present the following case in accordance with the CARE reporting checklist (available at https://shc.amegroups.com/article/view/10.21037/shc-22-2/rc).
Case presentation
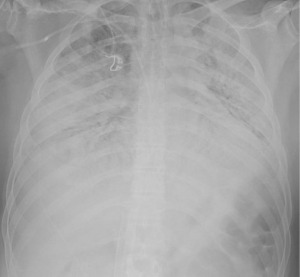
A 43-year-old male patient without chronic comorbidities, except for allergic asthma and hypothyroidism, presented to the rheumatologists with a 6-month history of arthralgia and weakness in July 2020. Two months later, he was admitted to our hospital for acute and severe dyspnea. The chest computed tomography (CT) scan showed widespread ground glass opacities and bilateral parenchymal consolidations. In September 2020, the patient underwent a skin biopsy that showed a positivity to anti-MDA5 antibodies which led to the diagnosis of SSc. At this stage, a combined immunosuppressive treatment regimen was initiated, with intravenous cyclophosphamide (6 mg/kg/die), oral tacrolimus (6 mg/die) and methylprednisolone (1.5 mg/kg/day). However, a few days after hospital admission, the patient’s respiratory function started to deteriorate dramatically, requiring non-invasive ventilation (NIV) support. Despite NIV, the arterial blood gas results did not improve, and the patient remained critically ill. The clinical scenario was complicated by bilateral pneumothorax and severe pneumomediastinum, with subcutaneous cervical emphysema (Figure 1), that contraindicated the invasive mechanical ventilation therapy.
As a consequence, the patient was urgently referred to our intensive care unit for a V-V ECMO placement on September 25, 2020. We opted for an awake cannulation and conscious sedation in order to avoid the increase of positive chest pressures secondary to invasive mechanical ventilation. We went for a femoro-femoral configuration, due to the presence of bilateral cervical emphysema. A left 27-Fr drainage cannula was placed to the inferior vena cava, and a right 25-Fr return cannula was placed to the right atrium. We set the ECMO flow at 50 mL/kg per ideal body weight (IBW) and the sweep gas at 50 mL/kg IBW, with 100% FiO2 at the oxygenator. At that point we induced general anesthesia and intubated endotracheally. Immediately after we drained the neck emphysema via surgical neck incision, followed by insertion of percutaneous tracheostomy. Mechanical ventilation was set in the ultra-protective modality; 3 mL/kg IBW tidal volume, 8 breaths per minute respiratory rate, 40% FiO2 and 6 cmH2O positive end expiratory pressure (PEEP).
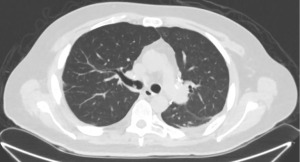
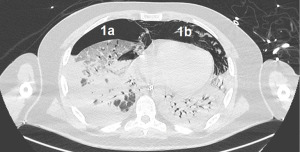
Despite adequate position of V-V ECMO cannulas, checked by transesophageal echocardiography, we were unable to obtain satisfactory oxygenation, with a peripheral SpO2 89–90%. Recirculation was appropriately excluded with blood gas analysis withdrawal in V-V ECMO out-let and in-let lines. As a consequence, we had to increase V-V ECMO flow by adding a 21-Fr cannula into the right internal jugular vein as a return cannula. With this configuration, we achieved a double femoral drainage with a higher ECMO flow and a more satisfactory oxygenation, and SpO2 raised up to 98%. However, despite one week of V-V ECMO therapy, ventilation parameters were not encouraging (plateau pressure >40 cmH2O, driving pressure >20 cmH2O, compliance of the respiratory system <15 cmH2O). A follow up chest X-ray showed a complete bilateral lung consolidation, with an estimated shunt fraction of 100% (Figure 2).
As a consequence, the initial rescue V-V ECMO therapy became a potential bridge to lung transplant. After having excluded neurological injuries as well as further major organ involvement (gastrointestinal tract and kidneys), the multidisciplinary lung transplant team of our hospital, which includes respiratory physician, thoracic and cardiac surgeons, anesthesiologist and intensivist care specialists, decided to list the patient for an urgent bilateral lung transplant on October 3, 2020. A suitable donor was found 1 week later. A bilateral sequential lung transplantation was performed via Clamshell incision. ECMO configuration was changed from V-V to central veno-arterial (V-A) ECMO, in order to support the heart and circulatory system during pulmonary artery cross clamping and guarantee adequate patient oxygenation during the different steps of surgery. At the end of the surgical procedure, the central V-A ECMO was switched to a peripheral femoro-femoral one with a semi-Seldinger technique, with the aim to off-load the patient’s pulmonary circulation and right ventricle.
The use of peripheral V-A ECMO after lung transplant is supported by Wien’s group that demonstrated its role in reducing the incidence of primary graft dysfunction (PGD) and right heart dysfunction in the early postoperative period (17). Adding a distal leg reperfusion cannula to the ECMO circuit in order to avoid peripheral ischemic complication is a routine practice in our local institution. The patient showed stable perfusion and ventilation parameters throughout the first 72 hours post-transplant day (PTD). Peripheral V-A ECMO was removed on PTD 3. Spontaneous breathing trials on tracheostomy were started on PTD 7. The patient was discharged to the ward on PTD 25, after having completed his passive and active physiotherapy program (Figure 3).
At this point, the main clinical concern remained the risk of the autoimmune disease relapse. Therefore, we repeated the autoimmune panel (especially anti-NMDA 5) before discharging the patient home. The results showed only a weak positivity 3 months after lung transplant, maybe because of the immunosuppressive protocol, similar to the one adopted in the pre-transplant period. The patient was successfully discharged home on PTD 92.
Follow-up was carried out according to the local post-transplant guidelines, which includes regular controls of blood gas exchange and spirometry. Outpatient gastroenterological consultations were also booked to monitor for esophageal dysfunction generally linked to the initial diagnosis of SSc.
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
The article reports the clinical management of a case of acute and severe lung complication of SSc unresponsive to conventional therapy with non-invasive mechanical ventilation and immunosuppressive drugs. The patient was positive to antiMDA5 antibodies that belong to a subtype of CTD potentially leading to a RPILD with high mortality rate (18). There are no specific recommendations about management of RPILD and with this case report we can demonstrate that V-V ECMO may be used as a bridge to recovery or to transplant, and contributes to the literature (13,14,16). Hoopes et al. also support the V-V ECMO therapy as a bridge to salvage lung transplant for refractory interstitial end stage lung disease (19).
Patients with autoimmune CTD and advanced pulmonary involvement can be considered suboptimal candidates for lung transplant due to their underlying medical complexity and potential surgical risk. Nevertheless, the International Society of Heart and Lung Transplantation (ISHL) consensus document suggests that patients with autoimmune CTD can be acceptable candidates if there are no extrapulmonary manifestations (20). Nevertheless, a lung transplant is not so frequent in a patient with an acute manifestation of ILD and without any chronic lung disease (21). The Italian Urgent Lung Transplantation Program (IULTp) was developed in November 2010, showing satisfactory results in this type of patients (22,23). In our Center we have already described successful urgent lung transplantation in two patients with acute distress respiratory syndrome (ARDS) and the diagnosis of acute fibrinous and organizing pneumonia (AFOP) (24). The patient described in this case report shares these criteria: younger than 50 years old, mechanically ventilated and supported by V-V ECMO.
The choice to schedule the patient for an urgent lung transplantation was supported by the clinical and radiological evidence of an unrecoverable native lung function after 1 week of V-V ECMO support. There are no recommendations for when to proceed with listing for transplantation in this scenario. According to the evidence reported in the literature, the mortality rate of short duration V-V ECMO (<14 days) is lower than the mortality rate of adults who require long duration V-V ECMO (>14 days), because it is associated with potentially lethal complications like neurological injury, nosocomial infections, renal dysfunction and, eventually, multi organ failure (MOF) (25). The irreversibility of lung damage was evaluated with daily monitoring of the lung compliance and intrapulmonary shunt, estimated by blood gas analysis from the arterial and pulmonary artery catheter. Subsequent chest X-ray, showing parenchyma persistently infiltrated throughout the whole lung and the current diagnosis of autoimmune CTD further supported the diagnosis of irreversible lung damage.
Once the patient went through a successful bilateral lung transplant under the careful management of an experienced multidisciplinary team, there were further concerns regarding how the immune process may potentially continue to target autoantigens and fibrotic pathways on the new graft. To mitigate this risk as well as to prevent graft rejection, immunosuppressive therapy was initiated, with no clinical evidence of autoimmune disease relapse on the transplanted lungs at 3 months follow up. In this clinical context, mild lung disease may appear in the allograft after several years, due to either allograft rejection or recurrent mild ILD. However, this does not appear to impact long term survival (26). Furthermore, De Cruz and Ross provided convincing data that lung transplant in patients with SSc but poor extrapulmonary manifestations has similar outcomes than in other non-rheumatologic pulmonary conditions (27).
The present case report supports the benefit of lung transplant in autoimmune CTD even in the context of acute end-stage respiratory failure. Long-term follow-up is not available in our report. Long-term follow-up might be useful to further understand the outcomes of bilateral lung transplantation in cases of acute respiratory failure in RPILD secondary to SSc. Further studies with a long-term follow up are needed to support this practice and to assess the clinical benefits of lung transplant in autoimmune CTD complicated by RPLID.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-22-2/rc
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-2/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-2/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Peoples C, Medsger TA Jr, Lucas M, et al. Gender differences in systemic sclerosis: relationship to clinical features, serologic status and outcomes. J Scleroderma Relat Disord 2016;1:177-240. [Crossref] [PubMed]
- Rubio-Rivas M, Royo C, Simeón CP, et al. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum 2014;44:208-19. [Crossref] [PubMed]
- Nikpour M, Baron M. Mortality in systemic sclerosis: lessons learned from population-based and observational cohort studies. Curr Opin Rheumatol 2014;26:131-7. [Crossref] [PubMed]
- Ingegnoli F, Ughi N, Mihai C. Update on the epidemiology, risk factors, and disease outcomes of systemic sclerosis. Best Pract Res Clin Rheumatol 2018;32:223-40. [Crossref] [PubMed]
- Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016;352:h6819. [Crossref] [PubMed]
- Panchabhai TS, Abdelrazek HA, Bremner RM. Lung Transplant in Patients with Connective Tissue Diseases. Clin Chest Med 2019;40:637-54. [Crossref] [PubMed]
- Richardson CB, Singer JP. Lung Transplantation for Scleroderma-related Lung Disease. Curr Respir Care Rep 2014;3:79-87. [Crossref] [PubMed]
- Gleason JB, Patel KB, Hernandez F, et al. Pulmonary Artery Dimensions as a Prognosticator of Transplant-Free Survival in Scleroderma Interstitial Lung Disease. Lung 2017;195:403-9. [Crossref] [PubMed]
- Chan EY, Goodarzi A, Sinha N, et al. Long-Term Survival in Bilateral Lung Transplantation for Scleroderma-Related Lung Disease. Ann Thorac Surg 2018;105:893-900. [Crossref] [PubMed]
- Pradère P, Tudorache I, Magnusson J, et al. Lung transplantation for scleroderma lung disease: An international, multicenter, observational cohort study. J Heart Lung Transplant 2018;37:903-11. [Crossref] [PubMed]
- Sef D, Verzelloni Sef A, Trkulja V, et al. Midterm outcomes of venovenous extracorporeal membrane oxygenation as a bridge to lung transplantation: Comparison with nonbridged recipients. J Card Surg 2022;37:747-59. [Crossref] [PubMed]
- Biscotti M, Gannon WD, Agerstrand C, et al. Awake Extracorporeal Membrane Oxygenation as Bridge to Lung Transplantation: A 9-Year Experience. Ann Thorac Surg 2017;104:412-9. [Crossref] [PubMed]
- Huang J, Liu C, Zhu R, et al. Combined usage of extracorporeal membrane oxygenation and double filtration plasmapheresis in amyopathic dermatomyositis patient with severe interstitial lung disease: A case report. Medicine (Baltimore) 2018;97:e10946. [Crossref] [PubMed]
- Szczepiorkowski ZM, Winters JL, Bandarenko N, et al. Guidelines on the use of therapeutic apheresis in clinical practice--evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher 2010;25:83-177. [Crossref] [PubMed]
- Marchiset A, Neuville M, Voiriot G, et al. High-Emergency Lung Transplantation for Interstitial Lung Disease Associated With Anti-MDA5 Dermatomyositis: A Case Report. Transplant Proc 2021;53:2613-5. [Crossref] [PubMed]
- Leclair V, Labirua-Iturburu A, Lundberg IE. Successful Lung Transplantation in a Case of Rapidly Progressive Interstitial Lung Disease Associated with Antimelanoma Differentiation-associated Gene 5 Antibodies. J Rheumatol 2018;45:581-3. [Crossref] [PubMed]
- Hoetzenecker K, Schwarz S, Muckenhuber M, et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J Thorac Cardiovasc Surg 2018;155:2193-206.e3. [Crossref] [PubMed]
- Vuillard C, Pineton de Chambrun M, de Prost N, et al. Clinical features and outcome of patients with acute respiratory failure revealing anti-synthetase or anti-MDA-5 dermato-pulmonary syndrome: a French multicenter retrospective study. Ann Intensive Care 2018;8:87. [Crossref] [PubMed]
- Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013;145:862-7; discussion 867-8. [Crossref] [PubMed]
- Crespo MM, Lease ED, Sole A, et al. ISHLT consensus document on lung transplantation in patients with connective tissue disease: Part I: Epidemiology, assessment of extrapulmonary conditions, candidate evaluation, selection criteria, and pathology statements. J Heart Lung Transplant 2021;40:1251-66. [Crossref] [PubMed]
- International Society for Heart and Lung Transplantation. Lung Transplantation. JHLT 2018;37:1155-206. Available online: https://ishltregistries.org/downloadables/slides/2018/lung_adult.pptx
- Boffini M, Venuta F, Rea F, et al. Urgent lung transplant programme in Italy: analysis of the first 14 months. Interact Cardiovasc Thorac Surg 2014;19:795-800; discussion 800. [Crossref] [PubMed]
- Schiavon M, Faggi G, Di Gregorio G, et al. Single-center experience in urgent lung transplantation program in a country with a shortage of donors: Does the end justify the means? Clin Transplant 2017; [Crossref] [PubMed]
- Campisi A, Dell'Amore A, Bertolaccini L, et al. Urgent lung transplantation in acute fibrinous and organizing pneumonia: a sliding door or a new perspective? Gen Thorac Cardiovasc Surg 2020;68:136-41. [Crossref] [PubMed]
- Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J 2017;63:60-7. [Crossref] [PubMed]
- Hinze AM, Lin CT, Hussien AF, et al. Longitudinal assessment of interstitial lung disease in single lung transplant recipients with scleroderma. Rheumatology (Oxford) 2020;59:790-8. [Crossref] [PubMed]
- De Cruz S, Ross D. Lung transplantation in patients with scleroderma. Curr Opin Rheumatol 2013;25:714-8. [Crossref] [PubMed]
Cite this article as: Benedetto M, Piccone G, Dolci G, Antonacci F, Salvaterra E, Baiocchi M. Urgent lung transplant in acute pulmonary scleroderma: a case report. Shanghai Chest 2022;6:39.