
Lung resection as part of multi-modality treatment for stage IV lung cancer
Introduction
Long-term outcomes for patients with lung cancer remain poor, with overall 5-year survival rates between 15–25% (1). This low figure is largely driven by the high incidence of stage IV disease at the time of diagnosis (1). Treatment for patients with stage IV lung cancer has traditionally been limited to palliative systemic anti-cancer therapy (SACT) alone. Despite the introduction of more sophisticated targeted therapies, 1-year survival for these patients remains less than 20%, and 5-year survival as low as 3–6% (2).
In recent times, the concept of high-grade treatment with a multi-modality approach in patients with stage IV non-small cell lung cancer (NSCLC) but a low total volume of disease has become increasingly recognised (3). This involves a combination of SACT and local treatment (e.g., surgery, radiotherapy) to the primary and metastatic sites and has been shown to provide survival benefit in comparison to SACT alone (4). The eighth edition of the Tumour Node Metastasis (TNM) staging classification for lung cancer defines oligometastatic lung cancer as a single metastasis in a single organ (5). Whilst there is emerging evidence of a survival benefit provided by the inclusion of stereotactic ablative radiotherapy (SABR) as part of the treatment strategy for patients with a range of solid malignancies in an oligometastatic state (6), there is a paucity of phase III randomised controlled trials on the role of thoracic surgery in oligometastatic lung cancer. Furthermore, no UK studies analysing outcomes in this patient group have been published. In this case series, we present our experience of stage IV lung cancer patients treated with surgical resection as part of a multi-modality approach. We present the following article in accordance with the STROBE reporting checklist (https://shc.amegroups.com/article/view/10.21037/shc-22-8/rc).
Methods
All consecutive patients with clinical stage IV NSCLC who underwent lung resection as part of multi-modality treatment between January 2012 and December 2018 at Manchester University NHS Foundation Trust (MFT) were included. MFT provides tertiary and quaternary level adult cardiothoracic and cardiopulmonary transplantation services for the northwest of England. It employs six consultant thoracic surgeons who service a region of 3.2 million people with approximately 2,500 lung cancers diagnosed every year. Our centre performs over 500 lung cancer resections per annum and utilises a dedicated cardiothoracic critical care unit for elements of post-operative care.
All patients with stage IV NSCLC undergo staging contrast computed tomography, positron emission tomography and cranial magnetic resonance imaging. Endobronchial ultrasound trans-bronchial needle aspiration is also performed to assess the mediastinum and enhance the accuracy of pre-operative staging. Our local policy has evolved over time, and in our current practice for patients with stage IV NSCLC deemed suitable for multi-modality treatment (low volume of primary and metastatic disease with adequate physiological reserve and agreeable to the complexities and uncertainties of high-grade treatment) we endeavour to commence SACT as the first treatment modality. Patients that are subsequently shown to have stable or responding disease are then considered for radical local treatment to the primary tumour and metastatic disease. The exception to this strategy is patients with cranial metastases. In these patients, radiotherapy to the brain is given prior to SACT or resection of the primary tumour.
Patients were defined as having either ‘oligometastatic disease’ (defined as a single metastasis in a single organ) or ‘polymetastatic disease’ (defined as ≥2 metastases). Case notes were reviewed to establish the planned sequencing and content of multi-modality treatment and whether this was in line with local policy as described above. Adherence to this planned treatment and whether all modalities of treatment were completed were recorded. All cases of NSCLC were pathologically confirmed pre-operatively, and post-operative staging was assigned based on the post-operative histological analysis according to the 8th edition of the TNM Classification for Lung Cancer.
The work was approved by the steering committee of the Northwest Clinical Outcomes Research Registry (NCORR), which has full ethical approval from the North West—Haydock NHS Health Research Authority (No. IRAS 260294). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Given the retrospective nature of the work, individual consent for this study was waived. Outcomes assessed were 30- and 90-day mortality and 1-, 2- and 3-year survival. The survival period was defined as the number of days from the date of surgery to the date of death. Survival data at 36 months was available for 100% of patients. A survival curve was generated using the Kaplan-Meier method. The start date of the survival curve is the date of surgery. All statistical analysis was undertaken using SPSS version 28 (SPSS, Inc., Chicago, IL, USA).
Results
The study comprised 19 patients with a mean age of 59.6 years (standard deviation ±15.2 years, range, 20–76 years). Patient characteristics are displayed in Table 1. All metastases were synchronous in nature and were present at the index diagnosis of lung cancer. Site of metastases included brain (n=3), liver (n=2), spine (n=2), adrenal gland (n=5) and thorax (n=7). One patient was epidermal growth factor receptor (EGFR) positive, and no patient was PDL1 positive. No patient had pre-operative evidence of N2 disease, whilst four patients were post-operatively upstaged to N2 staging.
Table 1
| Variable | Value |
|---|---|
| Age (years) (mean ± SD) | 59.6±15.2 |
| Male, n (%) | 8 (42.1) |
| PS score (median, IQR) | 1.0 (1.0–1.0) |
| % predicted DLCO (mean ± SD), % | 74.6±14.7 |
| BMI (kg/m2) (mean ± SD) | 28.7±5.8 |
| Anaemia, n (%) | 5 (26.3) |
| Diabetes mellitus, n (%) | 3 (15.8) |
| Hypertension, n (%) | 8 (42.1) |
| Smoking, n (%) | 14 (73.7) |
| Arrhythmia, n (%) | 1 (5.3) |
| Ischaemic heart disease, n (%) | 2 (10.5) |
| COPD, n (%) | 5 (26.3) |
| Right-sided resection, n (%) | 16 (84.2) |
| Resected segments (mean ± SD) | 4.3±2.1 |
| Thoracotomy, n (%) | 16 (84.2) |
| Extent of resection, n (%) | |
| Complex lobectomy | 3 (15.8) |
| Pneumonectomy | 2 (10.5) |
Anaemia: anaemia is defined as haemoglobin <120 g/L for women and <130 g/L for men as per World Health Organisation classifications; Complex lobectomy: bilobectomy or sleeve lobectomy or chest wall resection. SD, standard deviation; PS, performance status; IQR, interquartile range; DLCO, diffusion capacity of the lung for carbon monoxide; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
All three patients with intracranial metastases received local therapy to the brain first. From the remaining 16 patients, nine were initially treated with systemic therapy prior to local treatments (in line with local policy), of whom 77.8% (n=7/9) completed all treatment modalities. Only 28.6% (n=2/7) of the seven patients whose treatment strategy did not follow this agreed local policy completed all treatment modalities. Individual patient diagnoses, planned treatments, and completed treatments are provided in Table 2.
Table 2
| Patient | Pre-treatment TNM staging | Classification & site of metastases | Pathology and predictive markers | Proposed treatment strategy (in chronological order) | Completed treatments | Extent of lung resection | Additional comments regarding treatment | Pathological TNM staging | Survival |
|---|---|---|---|---|---|---|---|---|---|
| 1 | T3N0M1a | Polymetastases (multiple contralateral lung nodules) | Adenocarcinoma | Chemotherapy; lung resection | Chemotherapy; lung resection | Right lower bilobectomy | T3N0M1a | Died at 101 months |
|
| 2 | T2aN0M1b | Oligometastasis (adrenal ×1) | Squamous | Chemotherapy; adrenal resection; lung resection | Chemotherapy; adrenal resection; lung resection | Left pneumonectomy | T2aN1M1b | Died at 19 months |
|
| 3 | T4N0M1b | Oligometastasis (adrenal ×1) | Adenocarcinoma | Chemotherapy; lung resection; adrenal resection | Chemotherapy; lung resection; adrenal resection | Right upper sleeve lobectomy | T3N0M1b | Died at 15 months |
|
| 4 | T2aN0M1b | Oligometastasis (distant rib ×1) | Adenocarcinoma | Chemotherapy; lung resection; rib resection | Chemotherapy; lung resection; rib resection | Right upper lobectomy | T2aN0M1b | Alive at 97 months |
|
| 5 | T2aN0M1c | Polymetastases (cranial ×2) | Adenocarcinoma | SRS brain; lung resection; chemotherapy | SRS brain; lung resection | Right upper lobectomy | Not suitable for adjuvant therapy due to development of additional metastatic disease | T2aN2M1c | Died at 8 months |
| 6 | T2aN0M1b | Polymetastases (spine ×2) | Adenocarcinoma, EGFR +ve | Spine radiotherapy; spinal surgery; lung resection; TKI | Spine radiotherapy; spinal surgery; lung resection; TKI | Left lower lobectomy | T3N2M1b | Alive at 75 months |
|
| 7 | T3N1M1b | Oligometastasis (spine ×1) | Adenocarcinoma | Lung resection; chemotherapy; spinal surgery | Lung resection; chemotherapy | Right upper lobectomy | No decision regarding spinal surgery due to claustrophobia & inability to undergo MRI scan | T1bN1M1b | Died at 35 months |
| 8 | T2aN0M1b | Oligometastasis (adrenal ×1) | Squamous | Lung resection; adrenal resection; chemotherapy | Lung resection; adrenal resection | Right upper lobectomy | Not suitable for adjuvant therapy due to development of additional metastatic disease | T2aN1M1b | Died at 5 months |
| 9 | T3N1M1a | Oligometastasis (ipsilateral pleural effusion) | Squamous | Lung resection; chemotherapy | Lung resection; chemotherapy | Right lower lobectomy | MPE confirmed prior to surgery from pleural fluid cytology | T3N0M1a | Died at 20 months |
| 10 | T3N1M1b | Oligometastasis (adrenal ×1) | Adenocarcinoma | Chemotherapy; lung resection; adrenal resection | Chemotherapy; lung resection; adrenal resection | Right upper lobectomy | T2bN1M1b | Alive at 62 months |
|
| 11 | T4N0M1a | Oligometastasis (contralateral lung nodule x1) | Squamous | Lung resection; lung radiotherapy; chemotherapy | Lung resection; lung radiotherapy | Left pneumonectomy | Patient declined chemotherapy | T4N2M1a | Died at 6 months |
| 12 | T2aN1M1b | Oligometastasis (liver ×1) | Adenocarcinoma | Chemotherapy; liver resection; lung resection | Chemotherapy; liver resection; lung resection | Right upper lobectomy | T2aN2M1b | Died at 41 months |
|
| 13 | T2aN0M1c | Polymetastases (multiple liver nodules) | Adenocarcinoma | Chemotherapy; lung resection; liver radiotherapy or surgery | Chemotherapy; lung resection | Right upper lobectomy | No liver treatment due to increasing burden of hepatic metastases over time | T2aN0M1c | Alive at 47 months |
| 14 | T2aN1M1b | Oligometastasis (adrenal ×1) | Squamous | Chemotherapy; lung resection; adrenal resection | Chemotherapy; lung resection | Right lower lobectomy | Adrenal surgery replaced by palliative treatment due to development of additional metastatic disease | T1N0M1b | Died at 12 months |
| 15 | T1N0M1b | Oligometastasis (cranial ×1) | Adenocarcinoma | Cranial resection; brain radiotherapy; lung resection; chemotherapy | Cranial resection; brain radiotherapy; lung resection; chemotherapy | Right upper lobectomy | T2N0M1b | Died at 3 months |
|
| 16 | T2aN0M1a | Oligometastasis (contralateral lung nodule x1) | Adenocarcinoma | Lung resection; lung resection; chemotherapy | Lung resection; lung resection | Right lower lobectomy | Patient declined chemotherapy | T2aN0M1a | Alive at 44 months |
| 17 | T2bN0M1a | Oligometastasis (contralateral lung nodule x1) | Squamous | Lung resection; lung resection; chemotherapy | Lung resection; lung resection | Right upper lobectomy | Adjuvant chemotherapy not given: patient felt to be too high risk due to post-operative stroke | T3N0M1a | Alive at 43 months |
| 18 | T2aN0M1b | Oligometastasis (anterior chest wall ×1) | Adenocarcinoma | Chemotherapy; lung resection; chest wall resection | Chemotherapy; lung resection; chest wall resection | Right lower lobectomy & chest wall resection | T2aN0M1b | Died at 10 months |
|
| 19 | T4N0M1c | Polymetastases (multiple cranial nodules) | Adenocarcinoma | Cranial resection; brain radiotherapy; lung resection; chemotherapy | Cranial resection; brain radiotherapy; lung resection; chemotherapy | Right upper lobectomy | T2aN0M1c | Alive at 39 months |
TNM, tumour node metastasis; EGFR, epidermal growth factor receptor; SRS, stereotactic radiosurgery; TKI, tyrosine kinase inhibitor; MRI, magnetic resonance imaging; MPE, malignant pleural effusion.
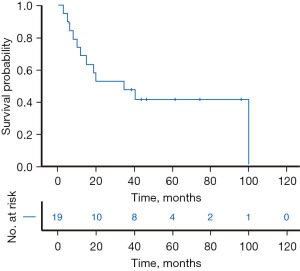
Mortality at 30 and 90 days was 0% (n=0). Median follow-up time was 35 months (range, 3–101 months). Observed 1-, 2- and 3-year survival rates were 73.7% (n=14), 52.6% (n=10) and 47.4% (n=9), respectively. Estimated median overall survival was 35 months (95% confidence interval 5–65 months). The Kaplan-Meier survival curve is shown in Figure 1.
Discussion
These results demonstrate that acceptable peri-operative outcomes and encouraging mid & long-term survival for highly selected patients with stage IV lung cancer (predominantly in an oligometastatic state) can be achieved. Our observed survival figures of 73.7%, 52.6% and 47.4% at 1, 2 and 3 years, respectively, are broadly similar to other reports described in the literature from across the world (2,7,8). Whilst it is apparent that these survival rates are markedly superior to those of patients undergoing non-surgical treatment, it is important to consider the inherent selection bias associated with this cohort, as only those patients with favourable patterns of metastatic disease and sufficient physiological reserve are selected to undergo ‘high-grade palliative treatment’ with radical resection of both primary and metastatic lesions as part of the multi-modality approach. The survival outcomes do, however, in the absence of phase III trial data, support our approach in these highly selected individuals.
This study has several limitations, foremost of which is its small sample size and single-centre nature. Due to the small cohort, it has not been possible to undertake further analysis to identify factors associated with survival. Moreover, as previously mentioned, unavoidable selection bias means that the results should be generalised to other settings with caution. In particular, this cohort will not include patients that were considered for multi-modality treatment but whose disease progressed through initial SACT rendering subsequent local therapy inappropriate. We also do not have data as to how many of the patients who underwent initial SACT in this study achieved a major pathologic response prior to undergoing additional treatment. Despite this selection bias, it is notable that almost half of all included patients were over 65 at the time of surgery and almost half had T3/4 or N1/2 disease, suggesting that the selection process was not inappropriately restrictive. Despite its small size, to the best of our knowledge, this is the first study to report outcomes after surgery for stage IV lung cancer in UK patients and is also among the most contemporary of all published datasets of this specific subset of patients. The heterogeneity of this small cohort should not be deemed a drawback of the study. Instead, we consider it a strength, as it is reflective of real-world practice and highlights the wide range of disease which falls under the umbrella classification of “stage IV NSCLC”.
Although previous publications have defined oligometastatic disease in a number of different ways, the current internationally accepted definition is a single metastasis in a single organ, as outlined in the most recent edition of the TNM staging classification for lung cancer (5). A small number of patients in this series (n=5/19) do not fall within this strict definition (defined in Table 2 as ‘polymetastases’) but are still considered as part of the oligometastatic spectrum as outlined in the consensus document authored by Guckenberger et al. (3).
The presence of N2 disease and undergoing a pneumonectomy have been shown to be amongst the strongest predictors of poor long-term outcomes in patients with advanced NSCLC (9). Patients with known N2 disease were selected for radical treatment in this cohort based on favourable factors such as age, location and number of metastatic lesions (10). Additionally, some patients with N2 disease were upstaged intra-operatively and were not known to have N2 disease prior to surgery. In general, the negative impact of these factors has been upheld in this study. Nevertheless, two patients with N2 disease enjoyed prolonged survival (died at 41 months and alive at 75 months), highlighting that each patient should be considered on an individual basis, and not turned down for high-grade treatment solely based on nodal status.
With regards to additional treatments in lung cancer, only one patient in this study had an EGFR mutation, meaning that our experience of the impact of molecular-based therapies on outcomes is limited. Nevertheless, there is strong evidence to suggest that treating EGFR positive patients with tyrosine kinase inhibitors (TKI) improves long-term survival (11) and is an important component of the multimodality approach to the treatment of lung cancer. The sole patient in our study receiving TKI treatment also underwent radical treatment to both the primary and metastatic sites of disease and remains alive at the time of writing.
The timing of resection of the primary tumour as part of the overall treatment plan for patients with synchronous oligometastatic disease remains poorly defined (12). Our preferred approach is to identify potentially suitable patients and offer SACT as a first-line treatment. Those patients who subsequently demonstrate an encouraging response to this treatment are then considered for further radical local treatments to treat both primary and metastatic lesions as part of the multi-modal approach. There also remains no consensus as to whether adopting either a metastasis-first or primary-first approach for radical local resection provides survival benefit (13). The exception to this strategy is cranial metastases, where local treatment of intracranial disease first is advocated, due to the poor penetration of SACT across the blood-brain barrier and the associated risk of disease progression (14). SACT and resection of the primary tumour are offered at a later date for those patients who respond well to the first stage of treatment. In this small dataset those patients with non-cranial metastases following our agreed policy of SACT first were more likely to complete all multi-modality treatment in comparison to those patients who did not proceed in line with this policy (77.8% completion versus 28.6% completion), lending further support to our preferred approach.
We have demonstrated that appropriately selected patients with low volume stage IV lung cancer in the UK can safely undergo therapeutic lung resection as part of high-grade palliative multi-modality treatment, with low peri-operative risk and encouraging rates of mid-term and overall survival. Whilst this study was too small to reliably identify factors associated with improved long-term survival, we believe that patients should ideally first undergo SACT and then proceed to local radical treatments if they demonstrate a satisfactory response to the initial systemic therapy. Additional studies are required to determine if these results are reproducible in larger UK patient cohorts and to clarify the optimum timing of resection of primary lung cancer and oligometastatic disease.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://shc.amegroups.com/article/view/10.21037/shc-22-8/rc
Data Sharing Statement: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-8/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-8/coif). MT serves as an unpaid editorial board member of Shanghai Chest from November 2021 to October 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The work was approved by the steering committee of the Northwest Clinical Outcomes Research Registry (NCORR), which has full ethical approval from the North West – Haydock NHS Health Research Authority (No. IRAS 260294). Given the retrospective and anonymised nature of the work, individual consent for this study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ganti AK, Klein AB, Cotarla I, et al. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol 2021;7:1824-32. [Crossref] [PubMed]
- Yang CJ, Gu L, Shah SA, et al. Long-term outcomes of surgical resection for stage IV non-small-cell lung cancer: A national analysis. Lung Cancer 2018;115:75-83. [Crossref] [PubMed]
- Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol 2020;21:e18-28. [Crossref] [PubMed]
- Uhlig J, Case MD, Blasberg JD, et al. Comparison of Survival Rates After a Combination of Local Treatment and Systemic Therapy vs Systemic Therapy Alone for Treatment of Stage IV Non-Small Cell Lung Cancer. JAMA Netw Open 2019;2:e199702. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Zhang C, Wang L, Li W, et al. Surgical outcomes of stage IV non-small cell lung cancer: a single-center experience. J Thorac Dis 2019;11:5463-73. [Crossref] [PubMed]
- Chikaishi Y, Shinohara S, Kuwata T, et al. Complete resection of the primary lesion improves survival of certain patients with stage IV non-small cell lung cancer. J Thorac Dis 2017;9:5278-87. [Crossref] [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [Crossref] [PubMed]
- Liao Y, Fan X, Wang X. Effects of different metastasis patterns, surgery and other factors on the prognosis of patients with stage IV non-small cell lung cancer: A Surveillance, Epidemiology, and End Results (SEER) linked database analysis. Oncol Lett 2019;18:581-92. [Crossref] [PubMed]
- Nan X, Xie C, Yu X, et al. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget 2017;8:75712-26. [Crossref] [PubMed]
- Richard PJ, Rengan R. Oligometastatic non-small-cell lung cancer: current treatment strategies. Lung Cancer (Auckl) 2016;7:129-40. [Crossref] [PubMed]
- Congedo MT, Cesario A, Lococo F, et al. Surgery for oligometastatic non-small cell lung cancer: long-term results from a single center experience. J Thorac Cardiovasc Surg 2012;144:444-52. [Crossref] [PubMed]
- Lanuti M. Surgical Management of Oligometastatic Non-Small Cell Lung Cancer. Thorac Surg Clin 2016;26:287-94. [Crossref] [PubMed]
Cite this article as: Taylor M, Whittaker G, Evison M, Booton R, Grant SW, Granato F. Lung resection as part of multi-modality treatment for stage IV lung cancer. Shanghai Chest 2022;6:32.


