A realist (re)view on surgical lung biopsy in interstitial lung disease: who, how, when and at what risk?
Introduction
Interstitial lung disease (ILD) is a heterogenous group of conditions causing scarring of lung parenchyma (1). It is divided into five groups of pathologies: idiopathic interstitial pneumonias (IIPs), sarcoidosis, hypersensitivity pneumonitis and autoimmune ILDs and other ILDs (Figure 1).
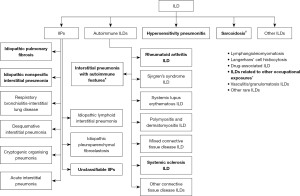
Idiopathic pulmonary fibrosis (IPF) and non-specific interstitial pneumonia (NSIP) both sit within the IIP category, which also includes other diagnoses such as cryptogenic organizing pneumonia (COP) and acute interstitial pneumonitis (Figure 1) (2). Both the treatments and prognoses of the different types of ILDs vary significantly. IPF, characterised by the usual interstitial pneumonia (UIP) pattern, is inevitably progressive with a poor and ultimately fatal prognosis (3,4). Research into optimum treatment continues. More recent trials have focused on anti-fibrotic agents Pirfenidone and Nintedanib (3-6).
In contrast, NSIP has a better prognosis (6), although much debate exists as to whether it is truly a separate entity as the histopathological pattern can be found in many other clinical and radiological contexts (6,7). Another condition with more favourable prognosis is sarcoidosis, which confers a 94% survival rate at 10 years and has a relatively well-established treatment protocol (8). Similarly, the trajectory of hypersensitivity pneumonitis can be altered with removal of hazards (9). Therefore, it is vital to obtain accurate diagnosis of such conditions.
Beyond the importance to treatment and prognosis, accurate diagnosis is vital for safeguarding the overall well-being of patients. Diagnostic uncertainty has been shown to cause lower satisfaction, confidence, and trust in the medical system. Consequently, it can heighten anxiety and encourage over-treatment, which in turn leads to increased hospital admissions and utilisation of resources (10). Logically, such uncertainty also decreases treatment efficacy (10,11), therefore feeding into a downward spiral of further anxiety, uncertainty and mistrust.
The role of surgical lung biopsy (SLB) in the context of ILD is to aid diagnosis of patients in whom a confident diagnosis cannot be made based on radiological and physiological findings. A key question for the multidisciplinary team is: how best to strike the balance between diagnostic benefit and risk profile of SLB in today’s complex medical arena (12)? To this end, this article uses realist review methodology (13) to enable decision-makers to reach a deeper understanding of the intervention and how it can be made to work most effectively. In short, it answers the question of “how, when and in whom to perform SLB?”
Methods
We reviewed key recommendations from prominent international societies in cardiothoracic surgery and respiratory medicine (1,2,14-16). Snowball sampling allowed for further exploration of the evidence underpinning these guidelines. We then used the realist review method (13) to build a best-fit approach to risk-benefit considerations for patients undergoing SLB.
Manchester University NHS Foundation Trust provides Thoracic Surgery services as well as a dedicated ILD Unit. It covers the Northwest of England, a region of 3.2 million people, which has the highest prevalence of ILD in the country. The dedicated specialist multi-disciplinary ILD team consisting of respiratory consultants, consultant thoracic radiologists, specialist nurses, physiologists and physiotherapists work in collaboration with six consultant thoracic surgeons as well as an experienced lung transplant team to ensure accurate diagnostic and management pathways for patients locally as well as nationally.
Discussion
Obtaining a diagnosis
Current diagnostic pathways in the UK and USA focus on a multi-disciplinary team (MDT) stepwise approach (1,14). In our centre, such an MDT consists of ILD specialist nurses, a team coordinator, respiratory physicians, histopathologists and speciality radiologists. The stepwise approach begins with an initial clinical evaluation consisting of history and examination. This allows assessment of domestic and occupational factors, as well as functional baseline. Simple tests include blood tests and chest X-ray. These may help to exclude alternative diagnoses and confirm interstitial pattern. Investigations include lung function tests (including gas transfer) and high-resolution computed tomography (HRCT). Lung function tests typically demonstrate a restrictive pattern with impaired gas transfer, while HRCT provides detailed imaging of the lung parenchyma. This initial diagnostic panel is sufficient for confident diagnosis in up to >90% of patients, although local variation needs to be taken into account (17,18).
Failing a definite diagnosis from the initial panel, further investigative options include bronchoalveolar lavage (BAL), transbronchial biopsy, lung cryobiopsy and surgical biopsy (Table 1).
Table 1
| SLB | Transbronchial lung biopsy | Lung cryo-biopsy | |
|---|---|---|---|
| Mortality | 2% elective, 16–20% non-elective | <0.1% | 0.2–2.7% |
| Complications | Prolonged air leak (5.9%), acute exacerbations (6.1%), bleeding (0.8%), severe bleeding (0.2%), neuropathic pain (4.5%), delayed wound healing (3.3%), respiratory infections (6.5%) | Pneumothorax (10%), prolonged air leak (6%) | Acute exacerbations (1.2%), prolonged air leak (13.2%), bleeding (5.2%), severe bleeding (0.7%), infection (0.7%) |
| Adequate specimen | 100% | 77.6% | 96% |
| Diagnostic yield | 89% | 43% | 83% |
SLB, surgical lung biopsy.
BAL may help to exclude alternative diagnoses such as chronic hypersensitivity pneumonitis, eosinophilic pneumonia and sarcoidosis (4). The complication rate is low, but not negligible. Therefore, it is reserved for selected patients whose radiological differential diagnoses include the above conditions but possess an indeterminate HRCT pattern (1,4,14).
Transbronchial biopsy may occasionally be considered as an alternative to SLB. Due to its relatively high complication rate, including air leak (6%) and pneumothorax (6%) and its low diagnostic yield with 64% of patients remaining undiagnosed, it is rarely indicated (1).
Transbronchial lung cryobiopsy (TBLC) has very recently been added to the guidelines as a potentially equivalent option to SLB (3). It has a higher diagnostic yield of 77–85% when compared to transbronchial biopsy (3). This increased diagnostic yield is balanced by the risk of bleeding (30%) and pneumothorax (9%) (1,3,19). However, severe bleeding, mortality, exacerbations, respiratory infections, and persistent air leak are rare (3,19). The emerging data regarding TBLC is of low quality and therefore the current guidelines make only a conditional recommendation to consider TBLC as an acceptable alternative to SLB in experienced centres that have taken steps to minimize risk and maximize diagnostic yield (3,20). Relative contraindications of the procedure are severe lung function derangement, moderate or severe pulmonary hypertension, high bleeding risk, and/or significant hypoxemia (17,19). However, emerging data suggests that TBLC may be an option in some high-risk patients, particularly when performed in higher volume centres (21,22). It is important to note that application of the histopathological criteria for UIP is more challenging with TBLC specimen because subpleural changes might not be represented and the potential for sampling error (3). Compared with SLB, TBLC is more likely to demonstrate a probable UIP pattern than a definite UIP pattern (23). However, combining UIP and probable UIP patterns at MDT level might result in comparable rates of diagnostic agreement for SLB and TBLC in patients with IPF (23).
Modern SLB is usually undertaken through a video-assisted thoracoscopic (VATS) approach under general anaesthetic. The main benefit of SLB in ILD is the possibility of obtaining an accurate diagnosis (1). Consequently, this allows for informed discussions with patients and within the MDT when considering prognosis and evaluating treatment options. An accurate diagnosis reduces patient’s uncertainty and anxiety (10,11). This is not only important when the diagnosis itself is in question, but can also be useful to inform prognosis in terms of disease stage, severity stratification and prediction of therapeutic response for a specific disease such as IPF (4).
SLB is indicated for patients in whom the MDT cannot make a diagnosis based on clinical features, PFTs and radiological findings (1,3,14). Currently there is insufficient evidence to reconsider this recommendation although it may be subject to a future review by the ERS taskforce (3). SLB obtains adequate samples in all patients and the diagnostic rate approaches 90%, compared to the 80% diagnostic yield of TBLC (1,3). Contraindications to surgery include an unacceptable risk for complications or mortality, severe pulmonary hypertension and DLCO of less than 25% after correction for haematocrit (1). Of note, SLB is more costly, invasive and time-consuming compared to TBLC (3). Figure 2 summarises the key benefits of SLB.
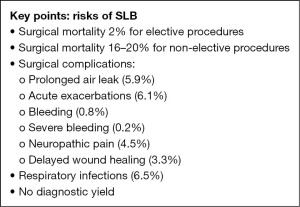
Risks of SLB
Once a patient is referred for consideration of SLB, the National Institute for health and Care Excellence (NICE) clinical guidance (14) highlights specifically that a discussion of risks and benefits must be had with the patient, outlining the potential benefit of being able to make a confident diagnosis versus uncertainty; as well as the risk of surgery, considering the patient’s clinical condition and risk factors. These may be categorised into surgical, anaesthetic and patient-specific factors (Table 2).
Table 2
| Surgical risk factors (17,18) |
| Thoracotomy |
| Anaesthetic risk factors (24) |
| Single lung ventilation |
| Increased pulmonary vascular resistance |
| Postoperative pain and use of opioids |
| Patient risk factors (17,18) |
| Male sex |
| Old age |
| Long-term oxygen therapy |
| Lung diffusion capacity <50% |
| Higher Charlson Comorbidity Index |
| Immunosuppressive treatment |
| Previous ICU admission |
ICU, intensive care unit
A major concern with SLB is mortality and morbidity. An older study (25) reviewed patients with histologically confirmed UIP undergoing SLB from 1986–1995. They reported a staggering 16.7% 30-day mortality. However, this series (25) is not representative of current surgical practice. In their study, 73% of patients underwent a thoracotomy for biopsy (due to the timeframe of inclusion) and includes high-risk patients who might not be offered surgical biopsy nowadays.
Recent studies report a slightly more modest 30-day mortality of around 2%; albeit including a more heterogenous patient group (17,18,26). A 30-day mortality of 2.4% and 90-day mortality of 3.9% were reported in a UK-based study of 2,820 patients (18). An American study reported in-hospital mortality of 1.7% for patients undergoing elective SLB (17). A Canadian study (26) reported overall 30-day mortality of 1.9% in a similar elective patient cohort. It is also worth noting that the 30-day mortality is significantly higher at 16–20% for non-elective procedures (1,17,18,26).
The frequency of surgical morbidity varies hugely across studies and patient cohorts with varying surgical approaches and patient fitness (1,17,18,25,27). Procedural complications have been summarised in a recent systematic literature review (1). They include pneumothorax (10.2%; 95% CI: 4.4–21.8%), prolonged air leak (6.1%; 95% CI: 33–39% ), acute exacerbations (6.1%; 95% CI: 5.1–7.3%), bleeding (0.8%; 95% CI: 0.4–1.7%), severe bleeding (0.2%; 95% CI: 0.04–1.2%), neuropathic pain (4.5%; 95% CI: 1.6–12.5%), delayed wound healing (3.3%; 95% CI: 2–5.4%) and respiratory infections (6.5%; 95% CI: 4.6–9.0%) (1) (Figure 3). Earlier studies (25,27) reported rates of respiratory failure after SLB as high as 26%, plus the need for postoperative intensive care admission (22%), intubation (13.6%) and tracheostomy (2.2%). However, this cohort included patients who might not undergo SLB nowadays due to poor fitness. In contrast, a recent cohort study from a tertiary centre reported no mortality in elective SLB with a major complication rate of 4% (28). Non-elective SLB in this study was associated with a higher (10%) mortality, as described previously (17,18).
Whilst SLB usually obtains adequate samples in all cases, current estimates of diagnostic yield suggest that up to 11% of patients continue to lack a formal diagnosis (1). This may be due to variation in interpretation of the histopathology (29), varying pathology in different areas of the lung (30) and the possibility of unclassifiable disease. Such patients would have undertaken a significantly risky procedure for no apparent benefit to themselves.
Anaesthetic considerations and risks may be considered in pre-, intra-, and post-operative categories. It is outside the scope of this paper to explore these in detail. The advent of Enhanced Recovery After Surgery (ERAS) pathways and guidelines have highlighted the importance of pre-operative patient engagement and education to the reduction of post-operative complications (31,32). General approaches to minimizing risk include smoking cessation and patient education. Certain centres advocate for further intensive pre-habilitation, but this depends on available resources, expertise, and healthcare networks (31). Intra-operative considerations are innumerable, and in general, maintenance of normal physiology is considered good practice (33). Two widely discussed considerations are one-lung ventilation (OLV) and patient positioning. OLV confers significant intraoperative respiratory stress. It increases the risk of hypoxia, hypercapnia, atelectasis, and ventilation/perfusion mismatch. But the risks are not confined to a single system since the increase in pulmonary vascular resistance may affect cardiovascular function. Poor positioning may result in pressure damage, as well as increased post-operative pain: already a major perioperative concern for thoracic surgery. Post-operative pain increases the risk of atelectasis and respiratory embarrassment. It may also increase opioid-use, which may further obtund the already fragile respiratory function. ERAS recommendations stress the importance of early mobilisation and physiotherapy to minimise post-operative respiratory complications (31,32). However, post-operative respiratory embarrassment often results from exacerbation of existing ILD (28).
Several large studies have highlighted patient-specific factors associated with perioperative mortality following SLB (17,18,26). The registry study from Ontario (26) identified advanced age, male sex, long-term oxygen therapy, earlier year of procedure and higher Charlson Comorbidity Index as significantly associated with a greater odds of 30-day post-operative mortality. Similar results were published from analyses of retrospective UK and the US registry data (17,18). Male sex, advanced age, higher comorbidity scores, and having a provisional diagnosis of IPF were identified as risk factors for 90-day mortality. Whilst there are technical limitations to analysing these large patient cohorts with medical interventions as far as two decades in the past, the uniformity of results across three countries and multiple surgical centres suggests some degree of internal and external validity. Whilst these risk factors have not been utilised in formal risk scoring, it might be important to consider them as part of the pre-operative assessment of potential surgical candidates.
Assessing risk
Generally, there is a lack of data for assessing the risk of patients with ILD specifically for SLB. An aggregate risk score has been proposed by Fibla et al. (27): the score, based on data from 311 patients, includes proportionally weighted variables according to their regression coefficient. These variables are open surgery, immunosuppressive treatment, age over 67 and previous ICU admission. According to the total score (0–6), patients are grouped into four risk categories A-D in ascending order of 90-day mortality risk: Class A, score 0 (2%); class B, score 1–2 (12%); class C, score 2.5–3 (40%); class D, score >3 (86%). However, discrepancies in the actual mortality rate between different units prevent external validity (34). Therefore, this scoring system has never been of practical clinical relevance.
A more commonly used risk scoring system is the ILD-GAP score, which predicts 1-, 2- and 3-year mortality in patients with ILD (35). This calculates overall mortality but is not specific to SLB. It is computed from the following variables: gender, age, forced vital capacity (FVC), diffusion capacity of the lung for carbon monoxide (DLCO). It has been shown to have good performance in all ILD subtypes, at all stages of disease severity. External validation has shown that the GAP score correlates well with 1-year mortality but not with 3-year mortality. An alternative scoring system is the du Bois score, which derives 1-year mortality for ILD patients. It is more specific but less sensitive compared to the GAP score (15). These systems may be used as a holistic approach towards suitability for surgery.
Anaesthetic risk assessment systems for SLB vary. An example is an adapted trimodal assessment consisting of respiratory mechanics, parenchymal function and cardiopulmonary reserve. Investigations for these include spirometry and gas exchange parameters, along with exercise testing, which may include shuttle walk or formal cardiopulmonary exercise testing (CPEX) (24,33). In a similar fashion, the surgical tripartite model, which is commonly used for risk assessment of patients with lung cancer requiring lung resection, considers the perioperative risk of death, risk of postoperative cardiac events and dyspnoea. Only if the patient is accepting of the risk in each category and the potential implications on their lifestyle can surgery be offered (16). Whilst the risk of perioperative death and major cardiovascular events are amongst the risks which ought to be discussed with patients undergoing SLB, the risk of dyspnoea is dictated by their primary disease progression rather than by the removal of a small amount of lung parenchyma for biopsy.
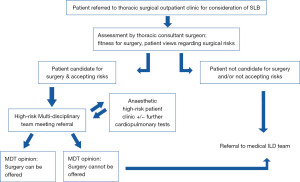
Whatever the system used, risk assessment plays an important part of informed decision-making processes between the MDT and the patient. In our unit, a comprehensive pre-operative assessment and discussion within a dedicated high-risk surgical MDT is arranged. The MDT team consists of the MDT coordinator, speciality nurses, consultant thoracic surgeons, consultant anaesthetists and consultant respiratory physicians. Where required, patients are referred to a dedicated high-risk anaesthetic clinic and further cardiopulmonary investigations undertaken (Figure 4).
Looking forward: who should be doing SLB and how?
Several studies have highlighted significantly higher 30-day mortality after non-elective compared to elective SLB (16–20% vs. 2%) (1,17,18,26,36). Unsurprisingly, better outcomes have been reported in high-volume centres (36). However, this statement warrants deeper exploration. In a retrospective registry study of over 3,000 procedures, the statistically significant reduction in mortality became insignificant when only elective cases were considered (P=0.08 vs. P=0.57). Yet, this is not to suggest that there are no outcome differences when considering volume of work in a centre. Indeed, when considering other surgical procedures, high-volume centres tend to perform better. This has been thought to result from more clinical experience in terms of patient assent, patient selection and perioperative care (37). Clearly more work needs to be done to better understand the nuances of outcomes and volume of work.
Given alternative biopsy techniques available such as transbronchial biopsy and lung cryobiopsy, along with the advances in biopsy sample interpretation using machine learning algorithms and RNA sequencing, should we be performing non-elective SLB at all considering its mortality risk (36)? Again, this rhetoric needs to be balanced with nuances in patient selection, local demographics, local training needs and available expertise, without exceeding tertiary capacities.
Since it has been shown that open SLB is associated with higher risk than minimally invasive techniques (17,18), a VATS approach is the gold standard recommended by NICE (14). The majority of SLB in the UK are performed using a VATS approach, in the lateral decubitus position, under general anaesthesia with single lung ventilation. The number of ports used varies from a single utility port to three-port technique. Whichever technique chosen, adequate sampling is mandatory and has been defined by the Fleischner society guidelines as samples from multiple lobes, at least 2–3 cm along the pleural axis and 1–2 cm deep (38). Regional techniques are performed pre-operatively for post-operative analgesia. These include paravertebral or erector-spinae plane blocks according to expertise of the anaesthetist. Along with this, intraoperative intercostal blocks and/or epi-pleural indwelling catheters for continuous infusion are inserted for post-operative analgesia.
A new development is the introduction of non-intubated, sedated, uniportal SLB (39,40). This has already demonstrated encouraging results but is not currently performed routinely in the UK. Key to this technique is effective teamwork between the surgical and anaesthetic teams. The use of regional and/or central neuraxial anaesthetic techniques such as erector spinae plane blocks and/or thoracic epidurals, along with surgical infiltration of local anaesthetic into the chest wall (subcutaneous and intercostal) allows for adequate anaesthesia for surgical access. Sedation is usually employed according to preferences of the anaesthetist to aid tolerability. Airway management may be achieved with spontaneous ventilation with nasal cannula, face masks or laryngeal mask airways (LMA) (41). Several small studies (39-41) have demonstrated lower 30-day mortality rates without compromising diagnostic yield. However, like intubated SLB, this requires very strict patient selection with a range of relative contraindications, such as obesity, complex airway, COPD with copious airway secretions, extensive adhesions, previous pulmonary resections, severe hypoxia or hypercapnia, inability to cooperate in the awake setting (41).
ILD is highly dimensional, diverse, and dynamic—a landscape which is reflected in how we navigate diagnostic options. The final decision of which investigative options to pursue, in what order, must be tailored to the clinical situation and priorities of individual patients, the local availability of services and expertise, and with a balanced and multi-disciplinary approach.
Conclusions
In summary, ILD is a heterogenous groups of conditions in terms of disease progression and prognosis. Accurate diagnosis is vital for tailored and effective therapy options. Mortality and morbidity vary significantly between centres and patient cohorts although recent studies suggest a more favourable risk-benefit ratio for elective SLB compared to non-elective SLB. Both surgical and non-surgical biopsy techniques have experienced progress with regards to safety and diagnostic yield. The complex balance between patient selection, urgency of surgery, volume of work, training needs and outcomes continues to evade equilibrium. Going forward, further research, continued monitoring, robust patient selection processes and multidisciplinary shared decision-making are some ways to optimise outcomes whilst minimizing risk. In the meantime, careful consideration of local expertise and availability of services, alongside robust MDT decision pathways, informed by multi-modal assessment methodologies, are key to decision-making which is patient-centred, clinically sound, and organizationally efficient.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcus Taylor and Felice Granato) for the series “Thoracic Surgery in High Risk Patients” published in Shanghai Chest. The article has undergone external peer review.
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-28/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-28/coif). The series “Thoracic Surgery in High Risk Patients” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Raghu G, Remy-Jardin M, Myers J, et al. The 2018 Diagnosis of Idiopathic Pulmonary Fibrosis Guidelines: Surgical Lung Biopsy for Radiological Pattern of Probable Usual Interstitial Pneumonia Is Not Mandatory. Am J Respir Crit Care Med 2019;200:1089-92. [Crossref] [PubMed]
- Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018;27:180076. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic Pulmonary Fibrosis (an Update) and Progressive Pulmonary Fibrosis in Adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2022;205:e18-47. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2071-82. [Crossref] [PubMed]
- du Bois R, King TE Jr. Challenges in pulmonary fibrosis x 5: the NSIP/UIP debate. Thorax 2007;62:1008-12. [Crossref] [PubMed]
- Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am J Surg Pathol 1994;18:136-47. [Crossref] [PubMed]
- Spagnolo P, Rossi G, Trisolini R, et al. Pulmonary sarcoidosis. Lancet Respir Med 2018;6:389-402. [Crossref] [PubMed]
- Salisbury ML, Myers JL, Belloli EA, et al. Diagnosis and Treatment of Fibrotic Hypersensitivity Pneumonia. Where We Stand and Where We Need to Go. Am J Respir Crit Care Med 2017;196:690-9. [Crossref] [PubMed]
- McGovern R, Harmon D. Patient response to physician expressions of uncertainty: a systematic review. Ir J Med Sci 2017;186:1061-5. [Crossref] [PubMed]
- Meyer AND, Giardina TD, Khawaja L, et al. Patient and clinician experiences of uncertainty in the diagnostic process: Current understanding and future directions. Patient Educ Couns 2021;104:2606-15. [Crossref] [PubMed]
- Cottin V, Tomassetti S, Valenzuela C, et al. Integrating Clinical Probability into the Diagnostic Approach to Idiopathic Pulmonary Fibrosis: An International Working Group Perspective. Am J Respir Crit Care Med 2022;206:247-59. [Crossref] [PubMed]
- Pawson R, Greenhalgh T, Harvey G, et al. Realist review--a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy 2005;10:21-34. [Crossref] [PubMed]
- NICE. CG 163: Idiopathic pulmonary fibrosis in adults: diagnosis and management: National Institute for Clinical Excellence. Available online: https://www.nice.org.uk/guidance/cg163/chapter/1-recommendations#diagnosis-2
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008;63:v1-58. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [Crossref] [PubMed]
- Hutchinson JP, Fogarty AW, McKeever TM, et al. In-Hospital Mortality after Surgical Lung Biopsy for Interstitial Lung Disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016;193:1161-7. [Crossref] [PubMed]
- Hutchinson JP, McKeever TM, Fogarty AW, et al. Surgical lung biopsy for the diagnosis of interstitial lung disease in England: 1997-2008. Eur Respir J 2016;48:1453-61. [Crossref] [PubMed]
- Aburto M, Pérez-Izquierdo J, Agirre U, et al. Complications and hospital admission in the following 90 days after lung cryobiopsy performed in interstitial lung disease. Respir Med 2020;165:105934. [Crossref] [PubMed]
- Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial Cryobiopsies for the Diagnosis of Diffuse Parenchymal Lung Diseases: Expert Statement from the Cryobiopsy Working Group on Safety and Utility and a Call for Standardization of the Procedure. Respiration 2018;95:188-200. [Crossref] [PubMed]
- Bondue B, Schlossmacher P, Allou N, et al. Trans-bronchial lung cryobiopsy in patients at high-risk of complications. BMC Pulm Med 2021;21:135. [Crossref] [PubMed]
- Ravaglia C, Wells AU, Tomassetti S, et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulm Med 2019;19:16. [Crossref] [PubMed]
- Troy LK, Grainge C, Corte T, et al. Cryobiopsy versus open lung biopsy in the diagnosis of interstitial lung disease (COLDICE): protocol of a multicentre study. BMJ Open Respir Res 2019;6:e000443. [PubMed]
- Lederman D, Easwar J, Feldman J, et al. Anesthetic considerations for lung resection: preoperative assessment, intraoperative challenges and postoperative analgesia. Ann Transl Med 2019;7:356. [Crossref] [PubMed]
- Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001;17:175-9. [Crossref] [PubMed]
- Fisher JH, Shapera S, To T, et al. Procedure volume and mortality after surgical lung biopsy in interstitial lung disease. Eur Respir J 2019;53:1801164. [Crossref] [PubMed]
- Fibla JJ, Brunelli A, Cassivi SD, et al. Aggregate risk score for predicting mortality after surgical biopsy for interstitial lung disease. Interact Cardiovasc Thorac Surg 2012;15:276-9. [Crossref] [PubMed]
- Pastre J, Khandhar S, Barnett S, et al. Surgical Lung Biopsy for Interstitial Lung Disease. Safety and Feasibility at a Tertiary Referral Center. Ann Am Thorac Soc 2021;18:460-7. [Crossref] [PubMed]
- Nicholson AG, Fulford LG, Colby TV, et al. The relationship between individual histologic features and disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2002;166:173-7. [Crossref] [PubMed]
- Monaghan H, Wells AU, Colby TV, et al. Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. Chest 2004;125:522-6. [Crossref] [PubMed]
- Gao S, Barello S, Chen L, et al. Clinical guidelines on perioperative management strategies for enhanced recovery after lung surgery. Transl Lung Cancer Res 2019;8:1174-87. [Crossref] [PubMed]
- Piccioni F, Droghetti A, Bertani A, et al. Recommendations from the Italian intersociety consensus on Perioperative Anesthesia Care in Thoracic surgery (PACTS) part 1: preadmission and preoperative care. Perioper Med (Lond) 2020;9:37. [Crossref] [PubMed]
- McCall P, Steven M, Shelley B. Anaesthesia for video-assisted and robotic thoracic surgery. BJA Educ 2019;19:405-11. [Crossref] [PubMed]
- Rotolo N, Imperatori A, Poli A, et al. Assessment of the aggregate risk score to predict mortality after surgical biopsy for interstitial lung disease†. Eur J Cardiothorac Surg 2015;47:1027-30; discussion 1030. [Crossref] [PubMed]
- Ryerson CJ, Vittinghoff E, Ley B, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP model. Chest 2014;145:723-8. [Crossref] [PubMed]
- Hutchinson J, Hubbard R, Raghu G. Surgical lung biopsy for interstitial lung disease: when considered necessary, should these be done in larger and experienced centres only? Eur Respir J 2019;53:1900023. [Crossref] [PubMed]
- Al-Sahaf M, Lim E. The association between surgical volume, survival and quality of care. J Thorac Dis 2015;7:S152-5. [PubMed]
- Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis - Authors' reply. Lancet Respir Med 2018;6:e7. [Crossref] [PubMed]
- Kim TH, Cho JH. Nonintubated Video-Assisted Thoracoscopic Surgery Lung Biopsy for Interstitial Lung Disease. Thorac Surg Clin 2020;30:41-8. [Crossref] [PubMed]
- Souza JM, Pereira IRPD, Borgmann AV, et al. Uniportal surgical biopsy, without orotraqueal intubation, without thoracic drainage in intersticial pulmonary disease: initial results. Rev Col Bras Cir 2021;48:e20202914. [Crossref] [PubMed]
- Bedetti B, Patrini D, Bertolaccini L, et al. Uniportal non-intubated thoracic surgery. J Vis Surg 2018;4:18. [Crossref] [PubMed]
Cite this article as: Brunswicker A, Tan MZY, Garner M, Rammohan K. A realist (re)view on surgical lung biopsy in interstitial lung disease: who, how, when and at what risk? Shanghai Chest 2022;6:36.