
Role of segmentectomy for lung metastases from colorectal cancer: latest insights and technical developments—a review
Introduction
What is the role of lung metastasectomy in the management of lung metastases from colorectal cancer (CRC)?
CRC is one of the major cancers worldwide (1,2). In addition, approximately 20% of all patients with CRC are diagnosed with distant metastases, with the liver and lungs being the most common sites of metastases (35% and 5–15%, respectively) (3).
Metastatic CRC patients have been shown to have a very poor prognosis with a 5-year survival rate, approximately 20% (4). In general, chemotherapy is considered the standard care for stage IV cancer. During the last two decades, chemotherapies such as using cytotoxic agents and monoclonal antibodies against epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) and immunotherapy have been developed (4-6), and these agents could prolong progression-free and overall survival (7,8). On the other hand, surgical resection with curative intent could provide survival benefits for selected patients with lung metastases, with a 5-year survival rate of >50% (9). This benefit is based on the concept of oligometastasis, an intermediate state between limited primary cancers and multi-metastatic cancers, and was first introduced by Hallman et al. (10). In other types of solid tumors, including germ cell tumors, melanoma, sarcoma, gynecological, urological, upper gastrointestinal, thyroid, and kidney cancers, patients with oligometastases in the lung could also be expected to have high survival rates after lung metastasectomy (11-13). And occasionally, surgical management of lung metastases from CRC can be proposed as a palliative approach in patients with uncontrollable pain, massive hemoptysis, or retention pneumonia due to centrally located metastases.
Who will benefit from lung metastasectomy?
So far, researchers have tried to establish criteria for determining which aggressive lung metastasectomy is beneficial. In 1958, Ehrenhaft et al. (14) presented the first criteria for lung metastasectomy. In 1965, Thomford et al. (15) proposed the criteria for surgical candidates based on the analysis of outcomes of 221 lung resections in 205 patients between 1941 and 1962, as follows: (I) the patient is at a low risk for surgical intervention; (II) the primary malignancy is controlled; (III) there is no evidence of metastatic disease elsewhere; and (IV) radiological evidence of lung metastasis is limited to one lung. These criteria have been frequently referred to date. Rusch et al. (16) described additional factors in selecting patients for surgery. These factors were the ability to resect all metastatic deposits and lack of better alternative systemic therapies. They stated that the assurance that complete surgical resection could be performed was critical to achieving long-term survival. Kondo et al. (17) summarized these principal criteria and included additional minor criteria as follows: the existence of effective systemic chemotherapy as a combined modality, difficulty in differentiating metastases from primary lung cancer, and the presence of symptoms of lung metastasis such as pneumothorax and hemoptysis. The essential aspects of the selection criteria have not changed in the clinical guidelines of many countries (18-23), despite developments and innovations in the field of oncology, including those in diagnostic methods, medical and radiological treatments, and genomic knowledge. Therefore, clinicians may wonder whether a patient would benefit from radical surgical treatment. Several prognostic factors have been explored to improve the selection criteria and facilitate the decision-making process of clinicians, mostly by retrospective observational studies with a limited sample size. Zellweger et al. (24) reported an integrated summary of studies published between 2005 and 2015 on prognostic factors. They concluded that patients with isolated unilateral lung metastases with normal carcinoembryonic antigen (CEA) levels and no lymph node involvement might benefit the most from surgery. Moreover, the absence of diseases other than liver metastases may have an impact on overall survival. Shimizu et al. (25) revealed that patients with CRC with lung metastasis and a history of curative hepatic metastasectomy may benefit from lung metastasectomy. However, these prognostic factors are yet to be included in the guidelines as clear indications for surgical resection of lung metastases from CRC.
What are the concerns about lung metastasectomy?
One of the greatest concerns for surgeons is whether recurrence will occur after curative surgical resection, as local recurrence and progression can lead to death following lung resection for metastases from CRC (26). In general, wedge resection has long been accepted as an appropriate strategy (16,27), which is in contrast to the surgical strategy for early-stage non-small cell lung cancer (NSCLC) (28). The rationale for this approach is based on the following reasons: the potential for resecting multiple lesions while preserving postoperative respiratory function, the lack of data about the benefit of anatomical resections, and the potential for safety without postoperative complications. However, non-anatomical resection, such as wedge resection, might be associated with an increased risk of local recurrence (29). In addition, the safety of lung segmentectomy has been demonstrated by JCOG0802/WJOG4607L, which was the largest phase III trial showing the efficacy and safety of lung segmentectomy in early-stage NSCLC (30). Taken together, in selected patients, lung segmentectomy may provide a lower postoperative recurrence rate than wedge resection and may be safer than previously considered. In this review, we aim to describe the role of lung segmentectomy in lung metastases from CRC. In addition, we will refer to the latest preoperative planning and localization strategies for lesions in nonpalpable and deep regions.
The potential benefit of lung segmentectomy for lung metastases from CRC
The potential benefit of lung segmentectomy
Wedge resection is defined as non-anatomical resection of the lung parenchyma. This procedure does not reveal structures such as the bronchus and pulmonary vessels. This method is widely preferred for resection of lung metastases because it is less invasive, preserves lung function, and can be repeated in the event of local recurrence (31). Lung segmentectomy reveals structures of the hilum corresponding to the target segment; it is an uncommon procedure for the management of lung metastases, and its rate ranged from 3% to 23% in previous studies (32,33). The safety analysis in JCOG0802/WJOG4607L (34) revealed that complications [Common Terminology Criteria for Adverse Events (CTCAE) grade >2] occurred in 27.4% of the patients, while no mortality was noted in 552 patients who underwent lung segmentectomy. The most common complications were air leakage (36 patients, 6.5%) and atrial arrhythmia (19 patients, 3.4%). Notably, in the trial, the 30-day mortality rate was 0%. This may be attributed to the development of surgical techniques and intensive perioperative management in the past few decades. Hence, lung segmentectomy may be safe, with acceptable morbidity. Furthermore, segmentectomy has more potential advantages than wedge resection.
Segmentectomy enables the assessment of regional lymph nodes. Historically, lymph node sampling or dissection is not a common procedure for surgeons in the management of lung metastasectomy. Indeed, in the international registry of lung metastases based on 5,206 patients reported in 1997 (11), lymph node sampling was performed in only 4.6% of the patients. However, the incidence of nodal involvement is relatively high, between 12% and 44%, in patients with lung metastases from CRC (35,36). Several studies have revealed that the presence of lymph node metastasis is a significant negative prognostic factor with a hazard ratio (HR) ranging from 1.5 to 2.6 (24). In accordance with this evidence, Handy et al. summarized an expert consensus on lung metastasectomy and recommended that lymph node sampling/dissection should be considered in all cases (23).
In addition, when the tumor is so large that the surgical margins cannot be obtained sufficiently, or the metastases cannot be found during surgery in a deep location, segmentectomy assures a better surgical margin than a wedge resection. A major site of recurrence after wedge resection is the surgical margin, even if complete resection is performed macroscopically (29,37). The frequency of recurrence at the surgical margin has been reported to be between 3.9% and 27.9% (27,29,37-41). Depth (40), tumor size, and distance from the surgical margin (42-44) were reported as independent prognostic factors for recurrence after wedge resection of lung metastases. The definition of the sufficient length of surgical margin is controversial. Nelson et al. (42) retrospectively evaluated the association between tumor size and length of surgical margins in 335 patients. They revealed that a longer margin length decreased the risk of local recurrence (HR, 0.434 per additional cm in length; P=0.015), while a larger tumor size increased the risk (HR, 1.520 per additional cm in size; P=0.012). Moreover, they evaluated the influence of margin on tumor recurrence and demonstrated that a margin length of at least half the tumor length could decrease the recurrence rate to under 11% for tumors of 3 cm or less. The study also revealed that if a 1 cm margin could be obtained for a 1 cm tumor or if a 2 cm margin could be obtained for a 2 to 4 cm tumor, the recurrence rate would decrease by approximately half. An attempt should be made to obtain a margin of equal or greater length for a tumor measuring 1–2 cm in size and a 2 cm margin for a tumor measuring 2 cm or more in size to minimize the risk of local recurrence.
Spread through alveolar space (STAS) is also an important factor in local recurrence. STAS has been recognized as an invasion pattern in primary lung cancer. It is defined as the spread of lung cancer cells into air spaces in the lung parenchyma beyond the edge of the main tumor (45). In primary lung cancer, STAS has been recognized as an important negative prognostic factor. As for the surgical margin, Eguchi et al. reported that the recurrence rate in patients with STAS-positive tumors and who underwent sublobar resection was high regardless of the margin/tumor length ratio (46). On the other hand, Masai et al. reported that no local recurrence occurred with a surgical margin greater than 2 cm, regardless of other factors, including tumor size, surgical procedure, and the presence of STAS (47). In lung metastases, STAS has also been recognized as a poor prognostic factor (48,49). Welter et al. investigated the presence of satellite tumors in resected specimens of 17 lung metastases from CRC (50), which revealed that satellite tumors that could replace STAS were found within 7.4 mm around the nodule in 99.73% of cases. They also evaluated the association between the length of the surgical margin and local recurrence rate after lung metastasectomy in another study, which revealed that local recurrence occurred frequently with a surgical margin less than 7.0 mm (44). However, in their study, the presence of STAS did not influence the risk of local recurrence (P=0.239), as relapse occurred in only 7% of the cases. Takeda-Miyata et al. revealed that the presence of STAS in resected lung metastases was associated with worse overall survival (P=0.002) and tended to increase the relapse rate at the site of the surgical margin (P=0.051) (49). In addition, a tumor margin of less than 1 cm was an independent poor prognostic factor for local recurrence [HR, 6.36; 95% confidence interval (CI): 1.392–28.835; P=0.02]. STAS was observed in lung metastases from CRC, ranging from 15% to 40% of the cases (45,49,50), while there might be no association between tumor size and STAS (46). Taken together, considering the possibility of STAS, a surgical margin of at least 1 cm should be obtained, even for tumors of 1 cm or less. Interestingly, Sawabata et al. reported that in lung resection for primary lung cancer, the length of the surgical margin depended on the part of the lung that was resected (51). A sufficient margin could be obtained at the edge (e.g., lingular segment) of the lung rather than at the large ovoid face (e.g., basal segment). Based on these considerations, the surgeon should not only pay attention to how much margin can be obtained for the tumor but also whether the tumor is in an area where a sufficient margin is available.
As for postoperative pulmonary function, Kent et al. reported the long-term results of the ACOSOG Z4032 trial (52), showing that the type of sublobar resection (wedge resection vs. segmentectomy) did not have a significant impact on postoperative pulmonary function at any time from 3 to 24 months after lung resection in patients with early-stage NSCLC. In other words, segmentectomy does not necessarily reduce pulmonary function anymore than wedge resection.
Lobectomy or greater lung resection is sometimes considered for large and/or proximal site tumors. A prospective national survey was conducted in Spain (53,54) that registered 532 patients, and 1,050 lung resections were performed. A total of 104 patients underwent lobectomy or greater lung resection. In the multivariate analysis, lobectomy or greater lung resection was an independent risk factor for postoperative morbidity (odds ratio, 1.9; 95% CI: 1.1–4.6). While complete resection is one of the most important goals of lung metastasectomy, surgeons should also consider preserving as much postoperative pulmonary function as possible because metastases from CRC frequently recur in the lungs (39) and sometimes require repeated resection (55). Several studies have suggested that repeated lung resection was acceptable for selected patients because it could provide favorable survival, ranging from 55% to 79% (26,55,56). However, patients who undergo repeated resection must have maintained surgical tolerance, including good performance status and sufficient pulmonary function, after the first lung metastasectomy. Therefore, when wedge resection is considered inappropriate, segmentectomy should be planned instead of lobectomy, considering the possibility that repeat resection may be necessary.
Taken together, the extent of resection should be minimized while ensuring sufficient surgical margins to prevent marginal recurrence, and segmentectomy for lung metastases from CRC may provide greater benefits than wedge resection or lobectomy for selected patients.
Prognostic outcomes of lung segmentectomy for lung metastases from CRC
There is no conclusive evidence available yet of the clinical benefits of lung segmentectomy in the management of lung metastases from CRC. To date, only four studies have revealed the extent of lung resection in patients with lung metastases from CRC (39,57-59). A comparison between segmentectomy and wedge resection has been reported by Shiono et al. (39) and Renaud et al. (57).
Shiono et al. (39) investigated the survival and recurrence rates in wedge resection and segmentectomy for lung metastases from CRC. A total of 553 patients were included retrospectively, 98 of whom underwent segmentectomy. Segmentectomy was preferred to wedge resection for large tumors (segmentectomy vs. wedge resection: 18 vs. 14 mm, P<0.001). Postoperative complications occurred in 36 (6.5%) patients and were more frequent with segmentectomy than with wedge resection (14.3% vs. 5.3%, P=0.001). The most common complication was prolonged air leakage, with a significant difference in incidence (5.1% vs. 1.8%, P=0.048). On the other hand, the 5-year overall survival of the patients who underwent segmentectomy was higher than that of those who underwent wedge resection (80.1% vs. 68.5%, P value was not shown), whereas the multivariate analysis revealed that the type of surgical procedure was not a significant prognostic factor for overall survival (HR, 0.65; 95% CI: 0.38–1.05; P=0.08). Postoperative recurrence was observed in 325 patients (63.7%). Recurrence at the resection margin occurred more frequently in patients who underwent wedge resection than in those who underwent segmentectomy (7.3% vs. 2.0%, P=0.035). In the multivariate analysis, the type of surgical procedure was a significant prognostic factor for recurrence-free survival, unlike in overall survival (HR, 0.63; 95% CI: 0.44–0.87; P=0.005). The researchers suggested that differences in the impact of segmentectomy on overall survival and recurrence-free survival were influenced by lymph node evaluation. Lymph node evaluation was performed in 73.5% of segmentectomies and in 5.5% of wedge resections. However, as the number of patients who were positive for lymph node metastases was not specified, the effect of lymph node evaluation on the results was uncertain.
Renaud et al. (57) revealed that the type of resection did not affect the overall survival and time to lung recurrence in the absence of KRAS mutations but significantly affected these in the presence of KRAS mutations. They evaluated the association between KRAS mutations and the extent of lung resection (wedge resection and segmentectomy). Of the 168 patients, 95 (56.5%) harbored KRAS mutations, and segmentectomy was performed in 74 (44%) patients. The type of resection did not have a significant impact on median overall survival for patients with wild-type KRAS [segmentectomy vs. wedge resection: 98 (95% CI: 78.64–117.36) vs. 118 (95% CI: 95.08–140.93) months, P=0.67], whereas it improved the median overall survival in patients with KRAS mutations [101 vs. 45 months (95% CI: 30.86–59.14), P=0.02]. Similarly, time to pulmonary recurrence significantly improved in patients with KRAS mutations undergoing segmentectomy [50 (95% CI: 40.19–59.82) vs. 15 (95% CI: 6.59–23.4) months, P=0.01]. Notably, the resection margin recurrence rate was significantly higher for wedge resection than for segmentectomy in patients with KRAS mutations (4.8% vs. 54.2%, P=0.001), even though there was no significant difference in the surgical margin length between wedge resection and segmentectomy (17 vs. 14 mm, P=0.19). They suggested that this result could be explained by the “seed and soil” theory, which states that the cancer cell (the seed) is able to live and grow only in an appropriate environment (the soil), provided by Stephan Paget (60). In general, it is widely believed that CRC cells reach the lung via the inferior vena cava directly from the rectal or portal veins and accumulate at the end of the peripheral pulmonary artery because of their size (61). However, according to the “seed and soil” theory, CRC cells with KRAS mutations induce caspase-independent death in endothelial cells of the lung vasculature and contribute to CRC cell extravasation into the lungs by increasing lung endothelial permeability (62). This may be one reason why KRAS-mutant CRC cells metastasize along the anatomic vascular structures of the lung and may explain the benefit of anatomical resection, including segmentectomy, in lung metastases from CRC with KRAS mutations. To date, several retrospective studies have revealed that KRAS mutations might be associated with a higher risk of lung recurrence, shorter recurrence-free survival, and shorter overall survival (63-65), whereas Nelson et al. reported that KRAS mutation was not associated with the risk of local recurrence (42). Therefore, the influence of KRAS mutation on the extent of lung metastasectomy remains controversial.
The clinical benefits of lung segmentectomy in the early stages of NSCLC are well established. However, because the biology of metastatic lung tumors differs from that of primary lung cancer, it is difficult to extrapolate the advantages of segmentectomy in primary lung cancer to segmentectomy in metastatic lung cancer. It is desirable to identify factors that are helpful in determining the appropriate surgical procedure.
Surgical strategy for lung metastases from CRC: the practice of segmentectomy planning
Segmentectomy planning and localization techniques for tumors
For tumors in deep locations and/or large tumors in which wedge resection does not provide adequate surgical margins, lung segmentectomy should be preferred over lobectomy, considering the risk of postoperative morbidity (55). However, segmentectomy is a technically challenging procedure because of the complexity of bronchovascular anatomy with individual anomalies (66,67). To overcome this problem, software devices have been developed which reconstruct three-dimensional (3D) images of the bronchovascular anatomy. The usefulness of 3D images for preoperative simulation and intraoperative navigation has been reported in recent decades, and 3D images are becoming an essential tool for safe and accurate segmentectomy (68-70). Eguchi et al. reviewed the technical advances in segmentectomy for lung cancer and introduced the latest software for reconstructing 3D images with a focus on lung segmentectomy planning, named REVORAS (Ziosoft, Inc., Tokyo, Japan), which has been in use in clinical practice at our institution (71). The most obvious advantage of this software is the ability to create segmentectomy plans based on dividing the bronchus or pulmonary artery or with specified margin distances. Moreover, the surgeon can intraoperatively ensure that the bronchus and pulmonary vessels are divided using 3D images called “key parts for segmentectomy” (71) (Figure 1). Nakao et al. demonstrated the high accuracy of this software by comparing 3D images with operative findings (72). According to their study, 3D images could be reconstructed in a median of 116 seconds, while the concordance rate with operative findings was over 90%. In general, a contrast agent is required to obtain precise 3D images. However, we reported the accuracy of 3D images based on non-enhanced computed tomography (CT), revealing that with little effort and in a short time, accurate 3D images based on non-enhanced CT, comparable to 3D images based on contrast-enhanced CT, could be reconstructed (74th Annual Scientific Meeting of the Japanese Association for Thoracic Surgery, October 31–November 3, 2021). These advances in preoperative simulation technology enable surgeons to make segmentectomy planning easier and can help perform precise segmentectomy.
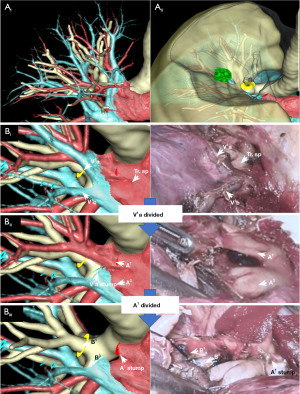
Intraoperative localization techniques for impalpable and deep-seated tumors are also important. This is because even if preoperative simulation is performed carefully, it is sometimes difficult to ensure that sufficient surgical margins have been obtained intraoperatively in cases of small impalpable tumors. Small tumors in deep sites can be identified using a bronchoscopic approach such as using microcoils (73), virtual-assisted lung mapping (VAL-MAP) (74), and contrast media (75). Although these bronchoscopic approaches cannot be monitored without fluoroscopy, radiofrequency identification (RFID) technology can identify the tumors to be resected without fluoroscopy and help ensure adequate surgical margins (76-78). RFID marking is a novel technique for tumor localization that has been approved for clinical use in Japan (SuReFInD, Hogy Medical Co., Ltd., Tokyo, Japan). RFID is a micro-tag with a nickel-titanium coil anchor (IC tag) as a marker, and it is placed in the bronchus within or close to the tumor through a bronchoscopic approach. After placement, a CT scan is obtained to confirm the existence of the RFID tag and to reconstruct 3D images to check the relationship between the tumor and the marker. During surgery, the surgeon can easily monitor the location of the marker using a detection device when dividing the lung parenchyma. After resection, the presence of an RFID marker in the resected specimen can be confirmed using an X-ray scan. Consequently, adequate surgical margins can be obtained for deep and impalpable tumors.
Strategy for lung metastasectomy at Shinshu University Hospital
Figure 2 shows a flowchart of the strategy for lung metastasectomy at Shinshu University Hospital. We usually hold multidisciplinary conferences four times to determine the indications. For lung metastasectomy, the patient criteria are principally based on the Clinical Practice Guideline of the Japan Society of Clinical Oncology (21) and the Japan Society for Cancer of the Colon and Rectum guidelines 2019 for the treatment of CRC (22). Simultaneously, we discuss which type of procedure is suitable in the given situation. In principle, surgical planning based on 3D CT is required for all patients. We reconstructed 3D CT-based resection planning using a novel software focused on segmentectomy, “REVORAS”. The size of the tumor and the possibility of STAS should be considered when determining the surgical margins based on previously mentioned studies (41,43,48). Wedge resection is preferred when the tumor is located adjacent to the visceral pleura with the expectation of obtaining a sufficient surgical margin, whereas anatomical resections such as segmentectomy and lobectomy should be considered when the tumor is in a deep site or it may be difficult to obtain adequate margins. As described before, segmentectomy is preferred over lobectomy in order to better preserve pulmonary function, reduce morbidity, and cater for the possibility of reoperation in the event of tumor recurrence at ipsilateral or contralateral sites.
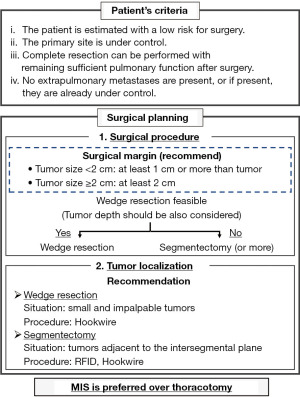
We sometimes use tumor localizing methods such as “RFID marking”, which is useful when the surgeons are concerned about the adequacy of the surgical margins owing to the tumor being adjacent to the intersegmental plane. “Hook wire marking” (79) is useful when the tumors are small and impalpable in the peripheral site. RFID marking is suitable when an appropriate target bronchus adjacent to the tumor can be found. Due care must be exercised in each method, as RFID markers can be displaced, and hook wires can cause pneumothorax (38%) and air embolism (0.6%) (79). Recently, thoracic minimally invasive surgeries such as video-assisted thoracic surgery and robot-assisted thoracic surgery (RATS) have been selected over thoracotomy, with growing evidence showing improved outcomes such as less postoperative pain, better quality of life, and reduced complications and non-cancer-related mortality (80-83). In particular, RATS can provide 3D high-definition visualization of the operative field and improve the precision of manipulations with tremor filtration and instrument dexterity (84). We have already combined the above novel technologies to provide safe complex segmentectomy and tailored the appropriate treatment to individual patients.
Conclusions
We reviewed published latest insights on lung metastasectomy for CRC. Few studies have shown the efficacy of lung segmentectomy for metastasectomy. However, with growing evidence, the safety and advantages of segmentectomy have been revealed mostly in early-stage NSCLC. Therefore, a well-planned, high-quality prospective study is urgently needed in the field of lung metastasectomy. Moreover, we focused on a novel segmentectomy technology. Segmentectomy has become easier and safer in the past decade with newly developed technologies, including RATS and precise 3D CT, and localization methods. Thoracic surgeons should be familiar with the latest technologies to provide appropriate treatments based upon a case to case evaluation.
Acknowledgments
We would like to thank Editage (https://www.editage.com/) for English language editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tomohiro Yazawa and Hitoshi Igai) for the series “Second Surgery” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-32/coif). The series “Second Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Cancer Information Service, National Cancer Center, Japan. Annual report of Hospital-based cancer registries. 2018. Accessed 24 May 2022. Available online: https://ganjoho.jp/reg_stat/statistics/data/dl/en.html
- Parnaby CN, Bailey W, Balasingam A, et al. Pulmonary staging in colorectal cancer: a review. Colorectal Dis 2012;14:660-70. [Crossref] [PubMed]
- Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021;325:669-85. [Crossref] [PubMed]
- Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019;125:4139-47. [Crossref] [PubMed]
- Franke AJ, Skelton WP, Starr JS, et al. Immunotherapy for Colorectal Cancer: A Review of Current and Novel Therapeutic Approaches. J Natl Cancer Inst 2019;111:1131-41. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Nordlinger B, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii1-9. [Crossref] [PubMed]
- Chiorean EG, Nandakumar G, Fadelu T, et al. Treatment of Patients With Late-Stage Colorectal Cancer: ASCO Resource-Stratified Guideline. JCO Glob Oncol 2020;6:414-38. [Crossref] [PubMed]
- Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg 2007;84:324-38. [Crossref] [PubMed]
- Hallman S, Weichselbaum R. Oligometastasis. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Petrella F, Diotti C, Rimessi A, et al. Pulmonary metastasectomy: an overview. J Thorac Dis 2017;9:S1291-8. [Crossref] [PubMed]
- Rena O, Papalia E, Oliaro A, et al. Pulmonary metastases from epithelial tumours: late results of surgical treatment. Eur J Cardiothorac Surg 2006;30:217-22. [Crossref] [PubMed]
- Ehrenhaft JL, Lawrence MS, Sensenig DM. Pulmonary resections for metastatic lesions. AMA Arch Surg 1958;77:606-12. [Crossref] [PubMed]
- Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [Crossref] [PubMed]
- Rusch VW. Pulmonary metastasectomy. Current indications. Chest 1995;107:322S-31S. [Crossref] [PubMed]
- Kondo H, Okumura T, Ohde Y, et al. Surgical treatment for metastatic malignancies. Pulmonary metastasis: indications and outcomes. Int J Clin Oncol 2005;10:81-5. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines for Colon Cancer, Version 1. 2022. Accessed 22 May 2022. Available online: https://www.nccn.org/guidelines/recently-published-guidelines
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. NICE guideline (NG151), Colorectal Cancer. 2020. Accessed 24 May 2022. Available online: https://www.nice.org.uk/guidance/ng151
- Japan Society of Clinical Oncology. Clinical Practice Guideline. 2007. Accessed 24 May 2022. Available online: http://jsco-cpg.jp/guideline/13.html#IV-2
- Japan Society for Cancer of the Colon and Rectum. JSCCR Guidelines 2019 for the Treatment of Colorectal Cancer. 2019. Accessed 24 May 2022. Available online: http://www.jsccr.jp/guideline/2019/index_guide.html
- Handy JR, Bremner RM, Crocenzi TS, et al. Expert Consensus Document on Pulmonary Metastasectomy. Ann Thorac Surg 2019;107:631-49. [Crossref] [PubMed]
- Zellweger M, Abdelnour-Berchtold E, Krueger T, et al. Surgical treatment of pulmonary metastasis in colorectal cancer patients: Current practice and results. Crit Rev Oncol Hematol 2018;127:105-16. [Crossref] [PubMed]
- Shimizu K, Ohtaki Y, Okumura T, et al. Outcomes and prognostic factors after pulmonary metastasectomy in patients with colorectal cancer with previously resected hepatic metastases. J Thorac Cardiovasc Surg 2019;157:2049-57.e1. [Crossref] [PubMed]
- Forster C, Ojanguren A, Perentes JY, et al. Is repeated pulmonary metastasectomy justified? Clin Exp Metastasis 2020;37:675-82. [Crossref] [PubMed]
- Davini F, Ricciardi S, Zirafa CC, et al. Lung metastasectomy after colorectal cancer: prognostic impact of resection margin on long term survival, a retrospective cohort study. Int J Colorectal Dis 2020;35:9-18. [Crossref] [PubMed]
- Aokage K, Yoshida J, Hishida T, et al. Limited resection for early-stage non-small cell lung cancer as function-preserving radical surgery: a review. Jpn J Clin Oncol 2017;47:7-11. [Crossref] [PubMed]
- Shiono S, Ishii G, Nagai K, et al. Predictive factors for local recurrence of resected colorectal lung metastases. Ann Thorac Surg 2005;80:1040-5. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Park JS, Kim HK, Choi YS, et al. Outcomes after repeated resection for recurrent pulmonary metastases from colorectal cancer. Ann Oncol 2010;21:1285-9. [Crossref] [PubMed]
- Berry MF. Role of segmentectomy for pulmonary metastases. Ann Cardiothorac Surg 2014;3:176-82. [PubMed]
- Gonzalez M, Brunelli A, Szanto Z, et al. Report from the European Society of Thoracic Surgeons database 2019: current surgical practice and perioperative outcomes of pulmonary metastasectomy. Eur J Cardiothorac Surg 2021;59:996-1003. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- Hamaji M, Cassivi SD, Shen KR, et al. Is lymph node dissection required in pulmonary metastasectomy for colorectal adenocarcinoma? Ann Thorac Surg 2012;94:1796-800. [Crossref] [PubMed]
- Renaud S, Alifano M, Falcoz PE, et al. Does nodal status influence survival? Results of a 19-year systematic lymphadenectomy experience during lung metastasectomy of colorectal cancer. Interact Cardiovasc Thorac Surg 2014;18:482-7. [Crossref] [PubMed]
- Higashiyama M, Kodama K, Takami K, et al. Intraoperative lavage cytologic analysis of surgical margins as a predictor of local recurrence in pulmonary metastasectomy. Arch Surg 2002;137:469-74. [Crossref] [PubMed]
- Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the "international registry of lung metastases". J Thorac Oncol 2011;6:1373-8. [Crossref] [PubMed]
- Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2017;51:504-10. [PubMed]
- Chung JH, Lee SH, Yi E, et al. Impact of resection margin length and tumor depth on the local recurrence after thoracoscopic pulmonary wedge resection of a single colorectal metastasis. J Thorac Dis 2019;11:1879-87. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Haney JC, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg 2009;87:1684-8. [Crossref] [PubMed]
- Nelson DB, Tayob N, Mitchell KG, et al. Surgical margins and risk of local recurrence after wedge resection of colorectal pulmonary metastases. J Thorac Cardiovasc Surg 2019;157:1648-55. [Crossref] [PubMed]
- Shiono S, Matsutani N, Hashimoto H, et al. Prospective study of recurrence at the surgical margin after wedge resection of pulmonary metastases. Gen Thorac Cardiovasc Surg 2021;69:950-9. [Crossref] [PubMed]
- Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO classification of tumors of the lung, pleura, thymus and heart. 4th ed. Lyon: International Agency for Research on Cancer, 2015:9-96.
- Eguchi T, Kameda K, Lu S, et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J Thorac Oncol 2019;14:87-98. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Shiono S, Ishii G, Nagai K, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg 2005;79:278-82; discussion 283. [Crossref] [PubMed]
- Takeda-Miyata N, Konishi E, Tanaka T, et al. Prognostic significance of spread through air spaces in pulmonary metastases from colorectal cancer. Lung Cancer 2020;149:61-7. [Crossref] [PubMed]
- Welter S, Theegarten D, Trarbach T, et al. Safety distance in the resection of colorectal lung metastases: a prospective evaluation of satellite tumor cells with immunohistochemistry. J Thorac Cardiovasc Surg 2011;141:1218-22. [Crossref] [PubMed]
- Sawabata N. Locoregional recurrence after pulmonary sublobar resection of non-small cell lung cancer: can it be reduced by considering cancer cells at the surgical margin? Gen Thorac Cardiovasc Surg 2013;61:9-16. [Crossref] [PubMed]
- Kent MS, Mandrekar SJ, Landreneau R, et al. Impact of Sublobar Resection on Pulmonary Function: Long-Term Results from American College of Surgeons Oncology Group Z4032 (Alliance). Ann Thorac Surg 2016;102:230-8. [Crossref] [PubMed]
- Embún R, Fiorentino F, Treasure T, et al. Pulmonary metastasectomy in colorectal cancer: a prospective study of demography and clinical characteristics of 543 patients in the Spanish colorectal metastasectomy registry (GECMP-CCR). BMJ Open 2013;3:e002787. [Crossref] [PubMed]
- Rodríguez-Fuster A, Belda-Sanchis J, Aguiló R, et al. Morbidity and mortality in a large series of surgical patients with pulmonary metastases of colorectal carcinoma: a prospective multicentre Spanish study (GECMP-CCR-SEPAR). Eur J Cardiothorac Surg 2014;45:671-6. [Crossref] [PubMed]
- Hachimaru A, Maeda R, Suda T, et al. Repeat pulmonary resection for recurrent lung metastases from colorectal cancer: an analysis of prognostic factors. Interact Cardiovasc Thorac Surg 2016;22:826-30. [Crossref] [PubMed]
- Hishida T, Tsuboi M, Okumura T, et al. Does Repeated Lung Resection Provide Long-Term Survival for Recurrent Pulmonary Metastases of Colorectal Cancer? Results of a Retrospective Japanese Multicenter Study. Ann Thorac Surg 2017;103:399-405. [Crossref] [PubMed]
- Renaud S, Seitlinger J, Lawati YA, et al. Anatomical Resections Improve Survival Following Lung Metastasectomy of Colorectal Cancer Harboring KRAS Mutations. Ann Surg 2019;270:1170-7. [Crossref] [PubMed]
- Hernández J, Molins L, Fibla JJ, et al. Role of major resection in pulmonary metastasectomy for colorectal cancer in the Spanish prospective multicenter study (GECMP-CCR). Ann Oncol 2016;27:850-5. [Crossref] [PubMed]
- Li H, Hu H, Li B, et al. What is the appropriate surgical strategy for pulmonary metastasis of colorectal cancer? Medicine (Baltimore) 2020;99:e21368. [Crossref] [PubMed]
- Auerbach R. Patterns of tumor metastasis: organ selectivity in the spread of cancer cells. Lab Invest 1988;58:361-4. [PubMed]
- Riihimäki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6:29765. [Crossref] [PubMed]
- Urosevic J, Garcia-Albéniz X, Planet E, et al. Colon cancer cells colonize the lung from established liver metastases through p38 MAPK signalling and PTHLH. Nat Cell Biol 2014;16:685-94. [Crossref] [PubMed]
- Renaud S, Romain B, Falcoz PE, et al. KRAS and BRAF mutations are prognostic biomarkers in patients undergoing lung metastasectomy of colorectal cancer. Br J Cancer 2015;112:720-8. [Crossref] [PubMed]
- Schweiger T, Hegedüs B, Nikolowsky C, et al. EGFR, BRAF and KRAS status in patients undergoing pulmonary metastasectomy from primary colorectal carcinoma: a prospective follow-up study. Ann Surg Oncol 2014;21:946-54. [Crossref] [PubMed]
- Ghidini M, Personeni N, Bozzarelli S, et al. KRAS mutation in lung metastases from colorectal cancer: prognostic implications. Cancer Med 2016;5:256-64. [Crossref] [PubMed]
- Shimizu K, Nagashima T, Ohtaki Y, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg 2016;64:604-11. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Ohtaki Y, Shimizu K. Anatomical thoracoscopic segmentectomy for lung cancer. Gen Thorac Cardiovasc Surg 2014;62:586-93. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Mogi A, et al. VATS segmentectomy: past, present, and future. Gen Thorac Cardiovasc Surg 2018;66:81-90. [Crossref] [PubMed]
- Shimizu K, Nakazawa S, Nagashima T, et al. 3D-CT anatomy for VATS segmentectomy. J Vis Surg 2017;3:88. [Crossref] [PubMed]
- Eguchi T, Sato T, Shimizu K. Technical Advances in Segmentectomy for Lung Cancer: A Minimally Invasive Strategy for Deep, Small, and Impalpable Tumors. Cancers (Basel) 2021;13:3137. [Crossref] [PubMed]
- Nakao M, Omura K, Hashimoto K, et al. Novel three-dimensional image simulation for lung segmentectomy developed with surgeons' perspective. Gen Thorac Cardiovasc Surg 2021;69:1360-5. [Crossref] [PubMed]
- Miyoshi T, Kondo K, Takizawa H, et al. Fluoroscopy-assisted thoracoscopic resection of pulmonary nodules after computed tomography--guided bronchoscopic metallic coil marking. J Thorac Cardiovasc Surg 2006;131:704-10. [Crossref] [PubMed]
- Sato M. Precise sublobar lung resection for small pulmonary nodules: localization and beyond. Gen Thorac Cardiovasc Surg 2020;68:684-91. [Crossref] [PubMed]
- Okumura T, Kondo H, Suzuki K, et al. Fluoroscopy-assisted thoracoscopic surgery after computed tomography-guided bronchoscopic barium marking. Ann Thorac Surg 2001;71:439-42. [Crossref] [PubMed]
- Yutaka Y, Sato T, Matsushita K, et al. Three-dimensional Navigation for Thoracoscopic Sublobar Resection Using a Novel Wireless Marking System. Semin Thorac Cardiovasc Surg 2018;30:230-7. [Crossref] [PubMed]
- Yutaka Y, Sato T, Zhang J, et al. Localizing small lung lesions in video-assisted thoracoscopic surgery via radiofrequency identification marking. Surg Endosc 2017;31:3353-62. [Crossref] [PubMed]
- Kojima F, Sato T, Takahata H, et al. A novel surgical marking system for small peripheral lung nodules based on radio frequency identification technology: Feasibility study in a canine model. J Thorac Cardiovasc Surg 2014;147:1384-9. [Crossref] [PubMed]
- Suzuki K, Shimohira M, Hashizume T, et al. Usefulness of CT-guided hookwire marking before video-assisted thoracoscopic surgery for small pulmonary lesions. J Med Imaging Radiat Oncol 2014;58:657-62. [Crossref] [PubMed]
- Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg 2014;148:637-43. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Darr C, Cheufou D, Weinreich G, et al. Robotic thoracic surgery results in shorter hospital stay and lower postoperative pain compared to open thoracotomy: a matched pairs analysis. Surg Endosc 2017;31:4126-30. [Crossref] [PubMed]
- Hristov B, Eguchi T, Bains S, et al. Minimally Invasive Lobectomy Is Associated With Lower Noncancer-specific Mortality in Elderly Patients: A Propensity Score Matched Competing Risks Analysis. Ann Surg 2019;270:1161-9. [Crossref] [PubMed]
- Veronesi G, Novellis P, Voulaz E, et al. Robot-assisted surgery for lung cancer: State of the art and perspectives. Lung Cancer 2016;101:28-34. [Crossref] [PubMed]
Cite this article as: Mishima S, Hamanaka K, Shimura M, Hara D, Matsuoka S, Miura K, Eguchi T, Shimizu K. Role of segmentectomy for lung metastases from colorectal cancer: latest insights and technical developments—a review. Shanghai Chest 2022;6:34.


