
Surgical management of pulmonary aspergilloma
Introduction
The aspergillus fungus can cause four types of lung disease: pulmonary aspergilloma, allergic bronchopulmonary aspergillosis, chronic necrotising pulmonary aspergillosis and invasive aspergillosis (1). This article will focus solely on pulmonary aspergillomas.
Aspergillomas form in a pre-existing cavity in the lung due to fungal colonisation of the space (2). There are a number of risk factors associated with their development. In the developing world they are mostly associated with tuberculosis, forming within a healed tuberculous (TB) cavity (3). In other settings they commonly occur in the immunosuppressed such as patient post transplantation, chemotherapy or those on immunosuppression for rheumatoid arthritis or inflammatory bowel disease. Aspergillomas have also been associated with sarcoidosis, bronchiectasis and cavitating tumors (3,4).
Belcher and Pulmmer classified aspergillomas into simple or complex in 1960 and this classification is still used today (5). Simple aspergillomas develop in thin walled lung cavities producing very localised disease. In these cases, the pleura is never involved. Complex cases are often more aggressive, they present with a more diffuse pattern of disease and often involve the adjacent pleura with a very thickened cavity due to repeated infections. Complex aspergillomas are commonly seen in TB patients and they often present with more symptoms and a reduced performance status. Complex aspergilloma patients can present significant challenge when deciding on surgical management as their operation is often more complex and they have poor physical status prior to surgery (6,7).
Most patient remain asymptomatic in the initial period of infection and they may be diagnosed through incidental findings on imaging. The most reported symptoms is haemoptysis which can be variable in it severity (3,8,9). It is thought to occur due to erosion into neighbouring blood vessels which are often hypertrophied and develop neo-vascularisation due to recurrent infection and inflammation (10). Patients also have symptoms related to their underlying lung disease such as dyspnoea and productive cough. Once there is contact with the pleura the patient may develop chest pain (10).
Diagnosis is usually achieved with radiological imaging. Once a fungus ball has developed there are characteristic radiological appearances of an opacity in a cavity surrounded by an air crescent. It is however important to recognise that similar appearances may occur with cavitating lung tumors, which need to be careful considered and excluded (11). Bronchoscopy can aid diagnosis with direct bronchoalveolar lavage to look for the aspergillus fumigatus additionally a biopsy of the bronchial tree mucosa can be performed. Some centres also perform antibody testing, Denning et al advised all patients should be tested for aspergillus fumigates immunoglobin G antibody as part of the diagnostic process (12).
Objectives
This articles aims to discuss the non-surgical surgical management of pulmonary aspergilloma in particular the indications and special considerations associated with operating on this unique disease.
Discussion
Narrative
Non-surgical treatment
The results of medical treatment for pulmonary aspergilloma have largely been disappointing and this is thought to be due to the cavity and fungal ball being avascular in nature (13). Instead, anti-fungals may be used to minimise clinical symptoms and this comes with a significant impact from side effects of these medications. Additional non-surgical treatments such as intra-cavity instillation of antifungal agents and bronchoscopic removal of the pulmonary aspergilloma under general anaesthetic have been suggested however these methods have been reported in small retrospective studies and are not routinely used in our practice (13-17). In the acute setting of haemoptysis patients may undergo bronchial artery embolization as a temporising measure to surgical intervention.
Surgical treatment
The aim of surgical intervention is to eradicate the fungal ball, resect the underlying cavity and diseased parenchyma in order to prevent life-threatening haemoptysis, invasive aspergillus disease, pulmonary fibrosis or renal amyloidosis due to chronic inflammation (18).
There is no agreed consensus on the indications or the timing of surgery and it has been associated with high morbidity and mortality rates (5,19-21). From 2000 onwards, the morbidity and mortality rates have much improved (22.5% and 2.2% respectively), which has tipped the balance more towards earlier surgical intervention (19,20,22). As a result, surgery is suggested for symptomatic patients with aspergilloma or lesions suspicious for a cavitating malignancy. Pre-operative optimisation of the patient is important with correcting anaemia, smoking cessation, prehabilitation with an exercise programme and management of superimposed infection with antibiotics. In addition, anti-fungal therapy prior to or in combination with surgery may offer additional protection against recurrence of aspergilloma after surgical resection (23). The administration of anti-fungals in the perioperative period appears to vary although there was not significant impact on recurrence when antifungals were continued post-operatively (23). It is important to note that anti-fungals are often poorly tolerated and need stopped due to significant side effects which may influence their pre- and post-operative use.
The surgical approach is through a posterolateral thoracotomy as the inflammatory nature of the condition often results in significant adhesions to the chest wall causing obliteration of the pleural space and dissection around the hilum maybe challenging due adhesions and collateral circulation. In addition, an extra pleural strip may be required to avoid opening the cavity and spilling of the aspergilloma contents into the chest cavity (3). Video-assisted thoracic surgery has more recently been reported in patients with localised aspergilloma with no parenchyma scaring and low volume lymphadenopathy with safe results (24). Anatomical lung resection is the gold standard treatment to allow full clearance of the infected lobe to prevent recurrence, additional wedge resection of adjacent lobes may be needed if the disease bridges the fissure. Some patients will not be able to tolerate anatomical lung resection due to poor physical reserves. In this case resection of the aspergilloma lesion, with apicolysis of the cavity and either a thoracoplasty or myoplasty to obliterate the resultant space may be required.
Surgical resection has been shown to greatly benefit patients who have had recurrent and significant haemoptysis associated with an aspergilloma (25). Jewkes et al. described a 5-year survival of 84% in this group who underwent surgical resection compared to 41% who were managed with medical therapy alone. In comparison there was no reported difference in survival between the groups in patients who are asymptomatic (75% versus 65% respectively) (25,26). These results remain similar in more recent studies with a 5-year survival rate post surgery of 85–93% (22,27). Despite this the rate of post-operative complication rate remains high (between 25–70%) with the complications most commonly being prolonged air leak, residual pneumothorax and bleeding which may need return to theatre (28,29).
After surgery the chance of recurrence of haemoptysis is low. In addition, recurrence rate of pulmonary aspergilloma is reported at 5–7% and is most commonly due to insufficient extent of parenchymal resection, the presence of further cavities in the lungs and continuation of immunosuppression (7,29).
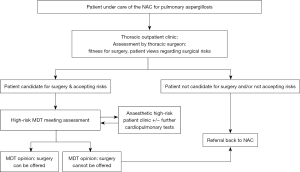
Given the high-risk nature of surgical treatment, a multi-disciplinary team (MDT) approach to assessing and treating these patients is crucial (Figure 1). In the UK, the National Aspergillosis Centre (NAC) has been commissioned by the Department of Health to provide long term care for patients with chronic pulmonary aspergillosis (CPA). If surgical treatment is considered, a referral is made from the NAC to the surgical outpatient clinic. Pre-operatively, the patient is assessed by a Consultant Thoracic surgeon. If the patient is deemed a potential surgical candidate and accepting of the risks of the procedure, they are referred to a dedicated high-risk thoracic surgery MDT (HRMDT). Members of the HRMDT include a surgical navigator, specialist nurses, thoracic surgeons, anaesthetists, and respiratory physicians. Further investigations, such as cardio-pulmonary exercise testing and a review in a high-risk anaesthetic clinic may be requested by the HRMDT. Patients with consensus from the MDT will be offered surgery. If a patient is not thought to be a surgical candidate, they will remain under the care of the NAC. Perioperatively, close cooperation with physicians from the NAC helps to guide antifungal treatment and future follow-up. The national centre provides coordinated specialist care for around 450 patients nationwide. Through cohorting these patients together in one centre their management can be coordinated by specialist and experienced clinicians including monitoring blood levels of anti-fungals to increase the compliance with medical therapy (30). In addition, this pool of patients facilitates high level research activity into treatment and management of all types of pulmonary aspergillus disease.
Summary
Pulmonary aspergilloma is a complex condition to manage. Previously surgical intervention was associated with high morbidity and mortality rates, however in recent years this has greatly reduced. The operations may be technically challenging and so it is still recommended that surgical intervention is reserved for symptomatic patients to prevent significant or life-threatening haemoptysis. It is crucial that surgical management is embedded within a multidisciplinary framework to manage this complex patient group.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marcus Taylor and Felice Granato) for the series “Thoracic Surgery in High Risk Patients” published in Shanghai Chest. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-5/coif). The series “Thoracic Surgery in High Risk Patients” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ma JE, Yun EY, Kim YE, et al. Endobronchial aspergilloma: report of 10 cases and literature review. Yonsei Med J 2011;52:787-92. [Crossref] [PubMed]
- Moodley L, Pillay J, Dheda K. Aspergilloma and the surgeon. J Thorac Dis 2014;6:202-9. [Crossref] [PubMed]
- Harmouchi H, Lakranbi M, Issoufou I, et al. Pulmonary aspergilloma: surgical outcome of 79 patients in a Moroccan center. Asian Cardiovasc Thorac Ann 2019;27:476-80. [Crossref] [PubMed]
- Kawamura S, Maesaki S, Tomono K, et al. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med 2000;39:209-12. [Crossref] [PubMed]
- Belcher J, Pulmmer N. Surgery in broncho-pulmonary aspergillosis. Br J Dis Chest 1960;54:335-6.
- Massard G, Roeslin N, Wihlm JM, et al. Pleuropulmonary aspergilloma: clinical spectrum and results of surgical treatment. Ann Thorac Surg 1992;54:1159-64. [Crossref] [PubMed]
- Daly RC, Pairolero PC, Piehler JM, et al. Pulmonary aspergilloma. Results of surgical treatment. J Thorac Cardiovasc Surg 1986;92:981-8.
- Muniappan A, Tapias LF, Butala P, et al. Surgical therapy of pulmonary aspergillomas: a 30-year North American experience. Ann Thorac Surg 2014;97:432-8. [Crossref] [PubMed]
- Chen QK, Jiang GN, Ding JA. Surgical treatment for pulmonary aspergilloma: a 35-year experience in the Chinese population. Interact Cardiovasc Thorac Surg 2012;15:77-80. [Crossref] [PubMed]
- Harmouchi H, Sani R, Issoufou I, et al. Pulmonary aspergilloma: from classification to management. Asian Cardiovasc Thorac Ann 2020;28:33-8. [Crossref] [PubMed]
- Estivals M, Barbe C, Rouleau V, et al. A lung cavity with an air crescent. Rev Mal Respir 2012;29:440-3. [Crossref] [PubMed]
- Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016;47:45-68. [Crossref] [PubMed]
- Ngo Nonga B, Bang GA, Jemea B, et al. Complex Pulmonary Aspergilloma: Surgical Challenges in a Third World Setting. Surg Res Pract 2018;2018:6570741. [Crossref] [PubMed]
- Krakówka P, Traczyk K, Walczak J, et al. Local treatment of aspergilloma of the lung with a paste containing nystatin or amphotericin B. Tubercle 1970;51:184-91. [Crossref] [PubMed]
- Giron J, Poey C, Fajadet P, et al. CT-guided percutaneous treatment of inoperable pulmonary aspergillomas: a study of 40 cases. Eur J Radiol 1998;28:235-42. [Crossref] [PubMed]
- Stather DR, Tremblay A, Dumoulin E, et al. A Series of Transbronchial Removal of Intracavitary Pulmonary Aspergilloma. Ann Thorac Surg 2017;103:945-50. [Crossref] [PubMed]
- Stather D, Tremblay A, MacEachern P, et al. Intracavity pulmonary aspergilloma removal using combined virtual ultrathin and rigid bronchoscopy – a case series. Chest 2013;144:817A.
- Liebler JM, Markin CJ. Fiberoptic bronchoscopy for diagnosis and treatment. Crit Care Clin 2000;16:83-100. [Crossref] [PubMed]
- Lee JG, Lee CY, Park IK, et al. Pulmonary aspergilloma: analysis of prognosis in relation to symptoms and treatment. J Thorac Cardiovasc Surg 2009;138:820-5. [Crossref] [PubMed]
- Karas A, Hankins JR, Attar S, et al. Pulmonary aspergillosis: an analysis of 41 patients. Ann Thorac Surg 1976;22:1-7. [Crossref] [PubMed]
- Kilman JW, Ahn C, Andrews NC, et al. Surgery for pulmonary aspergillosis. J Thorac Cardiovasc Surg 1969;57:642-7.
- Akbari JG, Varma PK, Neema PK, et al. Clinical profile and surgical outcome for pulmonary aspergilloma: a single center experience. Ann Thorac Surg 2005;80:1067-72. [Crossref] [PubMed]
- Setianingrum F, Rautemaa-Richardson R, Shah R, et al. Clinical outcomes of patients with chronic pulmonary aspergillosis managed surgically. Eur J Cardiothorac Surg 2020;58:997-1003. [Crossref] [PubMed]
- Chen QK, Chen C, Chen XF, et al. Video-assisted thoracic surgery for pulmonary aspergilloma: a safe and effective procedure. Ann Thorac Surg 2014;97:218-23. [Crossref] [PubMed]
- Shen C, Qiao G, Wang C, et al. Outcomes of surgery for different types of chronic pulmonary aspergillosis: results from a single-center, retrospective cohort study. BMC Pulm Med 2022;22:40. [Crossref] [PubMed]
- Jewkes J, Kay PH, Paneth M, et al. Pulmonary aspergilloma: analysis of prognosis in relation to haemoptysis and survey of treatment. Thorax 1983;38:572-8. [Crossref] [PubMed]
- Brik A, Salem AM, Kamal AR, et al. Surgical outcome of pulmonary aspergilloma. Eur J Cardiothorac Surg 2008;34:882-5. [Crossref] [PubMed]
- Lejay A, Falcoz PE, Santelmo N, et al. Surgery for aspergilloma: time trend towards improved results? Interact Cardiovasc Thorac Surg 2011;13:392-5. [Crossref] [PubMed]
- Kim YT, Kang MC, Sung SW, et al. Good long-term outcomes after surgical treatment of simple and complex pulmonary aspergilloma. Ann Thorac Surg 2005;79:294-8. [Crossref] [PubMed]
- Maghrabi F, Denning DW. The Management of Chronic Pulmonary Aspergillosis: The UK National Aspergillosis Centre Approach. Curr Fungal Infect Rep 2017;11:242-51. [Crossref] [PubMed]
Cite this article as: Garner M, Brunswicker A. Surgical management of pulmonary aspergilloma. Shanghai Chest 2023;7:4.


