
Perioperative management and surgical procedure for prolonged air leak: a clinical practice review
Introduction
Background
The most common postoperative complication after lung resection is an alveolar-pleural fistula, or air leak. Although most air leaks resolve spontaneously with chest tube drainage, the incidence of prolonged air leak (PAL) after lung cancer resection was 10% to 26% in past reports (1-3). According to guidelines and past reports, PAL is defined as air leak lasting for more than 5 to 7 days (1-7).
Rationale and knowledge gap
Although PAL is not life-threatening, it negatively affects other perioperative outcomes. Patients with PAL are more likely to have complications, including pneumonia and empyema, and prolonged hospital stay (1-6). British Thoracic Society guideline recommend observation for PAL with a chest tube for spontaneous closure of the fistula (7). In recent reports, because non-surgical management with chest tube drainage, autologous blood patch, chemical pleurodesis or endoscopic management has shown efficacy, surgical management is rarely the first choice for PAL. However, there are still some cases that require surgical repair. Foroulis et al. reported that surgical management of PAL was required in the immediate postoperative period in 0.8% of all general thoracic surgeries (8).
Objective
For fast recovery and prevention of pulmonary complications, it is necessary to establish proper indications and procedures for surgical management of PAL. The purpose of this paper is to help thoracic surgeons to determine the treatment strategy when treating difficult-to-treat postoperative PAL.
Methods
This is a clinical Practice Review for PAL after lung resection. In this review, we described the indications of surgical management for PAL, the surgical procedures and preoperative preparations. The PubMed, Web of Science, Embase and the Cochrane Library were searched for management of PAL, or procedures for management of air leak. Although there are many reports or reviews which describe management for postoperative PAL and some describe the indications for surgical management, few reports describe the detail of surgical procedures for reoperation for postoperative PAL, and this is the limitation of this review. So, we collected the reports which described procedures of surgical management to prevent postoperative air leak at first operation, and attempted to describe options of surgical treatment for reoperation of PAL. Some of the surgical procedures were described based on the authors’ experience. The images used are from cases experienced by the authors and are presented with patient consent and approval of our institution’s IRB (No. 456).
Evaluation and management for PAL
Evaluation of PAL requiring intervention
Volume and trend of air leak, and ineffective drainage are major factors that need to be considered for surgical or non-surgical management (1).
Massive air leak indicates a large alveolar-pleural fistula, which is unlikely to be cured by observation. There have been a few proposed classifications that attempted to quantify the severity of PAL in the postoperative setting. Cerfolio et al. reported a classification of PAL based on the amount of air leak; during forced expiration only (grade 1), expiration only (grade 2), inspiration only (grade 3), continuous air leak present during both inspiration and expiration (grade 4) (9). If the amount of air leak does not tend to decrease, it indicates that the alveolar-pleural fistula does not tend to heal, and intervention should be considered. Recently, digital drainage systems have been developed to provide real-time monitoring of air leaks. Goto et al. reported that persistent air flow greater than 20 mL/min at 36 hours postoperatively was highly predictive of PAL, with sensitivity and specificity of 91% and 73%, respectively (10).
Ineffective drainage is also a factor in PAL and causes complications such as pyothorax associated with PAL (1,2). If the remain lung full expansion is not achieved adequately with air leak or if subcutaneous emphysema is increasing, an additional drain should be inserted, and the drain suction pressure should be increased. Some intervention, especially surgical treatment, should be considered if space remains after additional drainage (8).
In addition to these major factors, past studies reported other factors to predict PAL (11,12). The European Society of Thoracic Surgeons (ESTS) score uses only 3 factors; gender (male), body mass index (BMI) <18.5 kg/m2 and forced expiratory volume 1.0 (FEV1) <80% and predicted 4 risk classes of PAL >5 days: PAL is expected to occur in 6.3% of cases with 0 factors, 9.9% of cases with 1 factor, 13% of cases with 2 factors, and 25% of cases with 3 factors. Such a scoring system is also an indicator of whether to intervene.
There are no clear guidelines or large study reports that described which intervention, surgical or non-surgical management, should be performed. Non-surgical interventions are generally minimally invasive, so this is often a priority. On the other hand, surgical management may be considered when non-surgical management is deemed ineffective based on the volume and trend of air leak, prior surgical procedure, and the patient’s comorbid risk factors. For example, massive air leaks as large as Grade D, failure of lung expansion after additional drainage, or when a bronchopleural fistula cannot be ruled out. Foroulis et al. reported a criteria for surgical management as follows (8): (I) failure of non-surgical maneuvers, such as repeated blood pleurodesis or position of a chest tube drain in the 2nd intercostal space in the midclavicular line; (II) failure of the remaining lung to fully expand after continuous suction; and (III) a trend of air leak obtained by the digital drainage system does not tend to heal during several days.
Non-surgical management for PAL
Non-surgical management options mainly include autologous blood patch, chemical pleurodesis, and endobronchial therapy (1).
Autologous blood patch may provide a simple and inexpensive treatment; 50 to 100 mL of peripheral venous blood is taken from the patient’s arm and injected through the chest tube into the pleural cavity (1). A recent review shows a high rate of success of more than 89% in patients with PALs following pulmonary resection (13). Although reported complications are rare, tension pneumothorax due to thrombus obstruction in the chest tube should be noted. It may be a first choice of intervention unless there’s a lung expansion failure or a massive air leak.
Chemical pleurodesis is to administer chemical agents which cause an inflammatory response into the pleural space (1,2). It allows for sealing of the pleural space and air leak, and prevention of recurrent pneumothorax. Common chemical agents include talc, doxycycline, tetracycline, minocycline, bleomycin, and OK-432 (mixture of a low-virulence strain of Streptococcus pyogenes incubated with benzylpenicillin). Although pleurodesis is an effective choice to management PAL, which successful rate is reported 63% to 95%, it requires direct apposition of the visceral and parietal pleura. Therefore, chemical pleurodesis should be performed only if there is no or only a small residual space. Otherwise, chemical pleurodesis should not be performed, as it may result in a lung that is unable to re-expand. In patients with underlying interstitial pneumonitis and older age, chemical pleurodesis is reported to be a risk factor of reduced lung function or acute respiratory distress syndrome (14,15). In such cases, surgical treatment would be considered in preference to non-surgical management.
There are several case series reports describing the success of endoscopic therapy for Watanabe spigots (16,17) and one-way valves placement (18-20). Both techniques are minimally invasive treatments that involve the insertion of a plug into the bronchus and are expected to be an alternative to surgery. Especially in recent years, the usefulness of implanting a one-way endobronchial valve has been widely reported (20). In both procedures, care must be taken to avoid infection and decreased lung function due to inhibition of the expansion of the remaining lung.
Evaluation of air leak site before second surgery
The main concern for surgical management of PAL is the possibility of pleural adhesion after the first operation or pleurodesis. Because severe pleural adhesions need extensive adhesiolysis, which may cause lung injury and secondary air leak, sometimes it is difficult to detect the original site of air leak during surgery for PAL (8,21). Although it is reported that the most common air leak sites are the suture line, interlobar fissure, and intersegmental plane, intraoperative detection of air leak sites needs to observe the whole pleural cavity. If the original air leak site can be identified before second surgery, unnecessary adhesiolysis can be avoided, and an access port can be easily placed to facilitate surgical manipulation.
Recently, the effectiveness of fluoroscopic thoracography has been reevaluated, and it was reported that in 73% of cases of pneumothorax surgery, air leak sites could be identified preoperatively (22). Three-dimensional identification of air leak sites by thoracography using computed tomography has also been reported (23-25). Figure 1 shows an example of a detected air leak site. To minimize surgical invasiveness and to ensure the closure of fistula, ensure closure of the fistula, it is advisable to detect the site of air leakage before second surgery. Identification of the air leak point may also be useful information in non-surgical management such as endobronchial treatment.
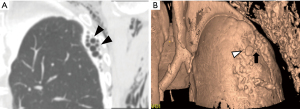
Surgical procedures for PAL
In the surgical management, it is difficult to suture the air leak site near the hilum, near the pleural defect due to adhesiolysis, and on emphysema. Such cases may need not only conventionally closure methods such as suturing or stapling, but also additional procedures including covering with various sealant products or biological tissues, and sometimes several methods are combined to control air leaks.
Fibrin glue has been used as the most common sealant to close air leaks and is considered effective (26). However, fibrin glue is very costly and has an intrinsic risk of allergy or viral and prion infection because it is made by allogenic human pooled plasma from multiple donors (27,28). To avoid these risks associated with allogenic fibrin glue, autologous fibrin glue may be a viable alternative, and its usage has been reported to be alternative to conventional fibrin glue (29). However, autologous fibrin glue requires preoperative blood collection. Recently, a polymeric biodegradable hydrogel sealant is reported as a safety and efficacy surgical sealant to seal intraoperative alveolar air leaks (30). This sealant stays in place and allows for the expansion and relaxation of the lung tissue until it biodegrades and is completely reabsorbed from the lung surface by 1 month after surgery. Because there are no clinical trials to compare each sealant, it is difficult to generalize about the efficacy of each individual sealant.
Polyglycolic acid (PGA) mesh is also used, sometimes in combination with fibrin glue to enhance the closure of air leak. For thorough sealing, application of multiple layers of PGA mesh has been reported (30). However, the use of PGA mesh may increase the risk of bacterial infections leading to a higher incidence of empyema (31). Because surgical management of PAL has its own risk of empyema due to prolonged drain placement, the use of PGA mesh should be considered only after confirming no signs of infection in the intraoperative findings.
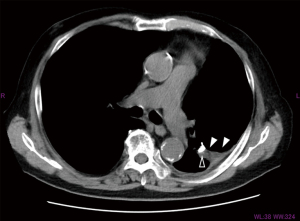
Covering air leak sites with biological tissue is an effective method without the risk of allergy or infection. A fat pad is a useful method because it is easy to make and easy to adjust the size to cover the air leak site (32-34). Figure 2 shows an example of covering the air leak site using free fat pad and various coverage sealants. As adipocytes are considered to have a low tolerance for ischemic conditions, a fat pad is ideally made from pericardium or thymus with pedicle. However, some cases demonstrate the difficulties associated with mobilization of flaps. Some studies reported that free fat pad, without pedicle, is also effective for sealing the fistula (33,34). Figure 3 shows the residual fat pad which was sutured on the air leak site 1 month after the surgery for PAL.
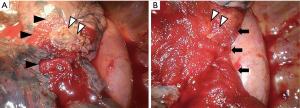
It is easier to make free fat pad than to make fat pad with pedicle. Even if it is difficult to make a fat pad from the thoracic cavity because of the adhesions due to PAL, it is possible to harvest free fat pad from the subcutaneous tissue and so on (34).
Muscular flaps are also used as biological material to cover air leak sites (35). An intercostal muscle flap is a common muscle flap used in thoracic surgery. However, adaptation of intercostal muscle flap to PAL depends on the extent of the damaged intercostal muscles in the first operation. Although latissimus and serratus muscle flaps can be used, these procedures require the extensive mobilization and it results in significantly increased invasiveness, especially postoperative pain and some degree of disability. Considering the invasiveness of the operation, muscular flaps can be used in few cases, except when there is a large residual space in the thoracic cavity that needs to be filled with muscle. Table 1 shows the advantage and disadvantage of each surgical procedure of biological tissue covering.
Table 1
| Biological tissue | Advantages | Disadvantages |
|---|---|---|
| Pericardium or thymus flap with pedicle | Can be made in the thoracic cavity | Mobilization, sometimes not available due to adhesion |
| Free fat pad | Easier to make, mobilization | Susceptible to ischemia |
| Intercostal muscle flap | Easier to make | Sometimes not available depending on first surgery |
| Latissimus and serratus muscle flaps | Covering the remained space | Highly invasiveness |
Conclusions
There have been no studies comparing various surgical procedures for PAL or, for that matter, even evidence of efficacy of surgical procedures for PAL. Therefore, in this review, it is not possible to present a standard surgical procedure that is reliably effective in each situation. However, regardless of the method, surgical management can directly approach and cover the air leak site. It is important to be prepared to use a series of methods until the air leak site is adequately covered and air leak disappears in a sealing test.
Acknowledgments
The content of this paper was advised by the Japanese Society of Clinical Research.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Tomohiro Yazawa and Hitoshi Igai) for the series “Second Surgery” published in Shanghai Chest. The article has undergone external peer review.
Peer Review File: Available at https://shc.amegroups.com/article/view/10.21037/shc-22-42/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://shc.amegroups.com/article/view/10.21037/shc-22-42/coif). The series “Second Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dugan KC, Laxmanan B, Murgu S, et al. Management of Persistent Air Leaks. Chest 2017;152:S0012-3692(17)30264-7.
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:S0003-4975(14)01921-3.
- Varela G, Jiménez MF, Novoa N, et al. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg 2005;27:329-33. [Crossref] [PubMed]
- Brunelli A, Xiume F, Al Refai M, et al. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest 2006;130:1150-6. [Crossref] [PubMed]
- Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg 2010;89:891-7; discussion 897-8. [Crossref] [PubMed]
- Attaar A, Luketich JD, Schuchert MJ, et al. Prolonged Air Leak After Pulmonary Resection Increases Risk of Noncardiac Complications, Readmission, and Delayed Hospital Discharge: A Propensity Score-adjusted Analysis. Ann Surg 2021;273:163-72. [Crossref] [PubMed]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii61-76. [Crossref] [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6:S21-31. [Crossref] [PubMed]
- Cerfolio RJ. Advances in thoracostomy tube management. Surg Clin North Am 2002;82:833-48. vii. [Crossref] [PubMed]
- Goto M, Aokage K, Sekihara K, et al. Prediction of prolonged air leak after lung resection using continuous log data of flow by digital drainage system. Gen Thorac Cardiovasc Surg 2019;67:684-9. [Crossref] [PubMed]
- Seder CW, Basu S, Ramsay T, et al. A Prolonged Air Leak Score for Lung Cancer Resection: An Analysis of The Society of Thoracic Surgeons General Thoracic Surgery Database. Ann Thorac Surg 2019;108:S0003-4975(19)31034-3.
- Gonzalez M, Karenovics W, Bédat B, et al. Performance of prolonged air leak scoring systems in patients undergoing video-assisted thoracoscopic surgery segmentectomy. Eur J Cardiothorac Surg 2022;62:ezac100. [Crossref] [PubMed]
- Hugen N, Hekma EJ, Claessens NJM, et al. Efficacy of an Autologous Blood Patch for Prolonged Air Leak: A Systematic Review. Ann Thorac Surg 2022;114:S0003-4975(21)01005-5.
- Shinno Y, Kage H, Chino H, et al. Old age and underlying interstitial abnormalities are risk factors for development of ARDS after pleurodesis using limited amount of large particle size talc. Respirology 2018;23:55-9. [Crossref] [PubMed]
- Maeyashiki T, Takamochi K, Matsunaga T, et al. Negative impact of chemical pleurodesis on postoperative pulmonary function for managing prolonged air leakage after segmentectomy. Gen Thorac Cardiovasc Surg 2021;69:707-15. [Crossref] [PubMed]
- Watanabe Y, Matsuo K, Tamaoki A, et al. Bronchial occlusion with endobronchial Watanabe spigot. Journal of Bronchology 2003;10:264-67.
- Himeji D, Tanaka GI, Fukuyama C, et al. Clinical Evaluation of Endoscopic Bronchial Occlusion with an Endobronchial Watanabe Spigot for the Management of Intractable Pneumothorax, Pyothorax with Bronchial Fistula, and Postoperative Air Leakage. Intern Med 2020;59:1835-9. [Crossref] [PubMed]
- Reed MF, Gilbert CR, Taylor MD, et al. Endobronchial Valves for Challenging Air Leaks. Ann Thorac Surg 2015;100:S0003-4975(15)00733-X.
- Cordovilla R, Torracchi AM, Novoa N, et al. Endobronchial valves in the treatment of persistent air leak, an alternative to surgery. Arch Bronconeumol 2015;51:S0300-2896(14)00174-4.
- Mukhtar O, Khalid M, Shrestha B, et al. Endobronchial valves for persistent air leak all-cause mortality and financial impact: US trend from 2012-2016. J Community Hosp Intern Med Perspect 2019;9:397-402. [Crossref] [PubMed]
- Cho S, Jheon S, Kim DK, et al. Results of repeated video-assisted thoracic surgery for recurrent pneumothorax after primary spontaneous pneumothorax. Eur J Cardiothorac Surg 2018;53:857-61. [Crossref] [PubMed]
- Watanabe K, Imamura N, Yusa J, et al. Pleurography (thoracography) for pleural fistulas: A case series. JTCVS Tech 2021;7:285-91. [Crossref] [PubMed]
- Nakanishi K, Shimotakahara A, Asato Y, et al. A new method to detect air leakage in a patient with pneumothorax using saline solution and multidetector-row spiral CT scan. Chest 2013;144:S0012-3692(13)60610-8.
- Watanabe T, Noda M, Okazaki T, et al. Preoperative saline-filled computed tomography thoracography for awake video-assisted thoracic surgery: report of three cases. Surg Today 2015;45:1579-82. [Crossref] [PubMed]
- Nakanishi K, Goto H, Ito T, et al. Novel imaging detailing the origins of a pneumothorax. Thorax 2018;73:85-7. [Crossref] [PubMed]
- Fabian T, Federico JA, Ponn RB. Fibrin glue in pulmonary resection: a prospective, randomized, blinded study. Ann Thorac Surg 2003;75:1587-92. [Crossref] [PubMed]
- Beierlein W, Scheule AM, Dietrich W, et al. Forty years of clinical aprotinin use: a review of 124 hypersensitivity reactions. Ann Thorac Surg 2005;79:741-8. [Crossref] [PubMed]
- Kawamura M, Sawafuji M, Watanabe M, et al. Frequency of transmission of human parvovirus B19 infection by fibrin sealant used during thoracic surgery. Ann Thorac Surg 2002;73:1098-100. [Crossref] [PubMed]
- Kawashima M, Kohno T, Fujimori S, et al. Feasibility of autologous fibrin glue in general thoracic surgery. J Thorac Dis 2020;12:484-92. [Crossref] [PubMed]
- Fuller C. Reduction of intraoperative air leaks with Progel in pulmonary resection: a comprehensive review. J Cardiothorac Surg 2013;8:90. [Crossref] [PubMed]
- Nomori H, Abe M, Sugimura H, et al. Triple-layer sealing with absorptive mesh and fibrin glue is effective in preventing air leakage after segmentectomy: results from experiments and clinical study. Eur J Cardiothorac Surg 2014;45:910-3. [Crossref] [PubMed]
- Porte HL, Jany T, Akkad R, et al. Randomized controlled trial of a synthetic sealant for preventing alveolar air leaks after lobectomy. Ann Thorac Surg 2001;71:1618-22. [Crossref] [PubMed]
- Shintani Y, Inoue M, Nakagiri T, et al. Use of free subcutaneous fat pad for reduction of intraoperative air leak in thoracoscopic pulmonary resection cases with lung cancer. Eur J Cardiothorac Surg 2014;46:324-6. [Crossref] [PubMed]
- Ikeda T, Sasaki M, Yamada N, et al. Controlling air leaks using free pericardial fat pads as surgical sealant in pulmonary resection. Ann Thorac Surg 2015;99:S0003-4975(14)02204-8.
- Pairolero PC, Arnold PG, Trastek VF, et al. Postpneumonectomy empyema. The role of intrathoracic muscle transposition. J Thorac Cardiovasc Surg 1990;99:958-66; discussion 966-8.
Cite this article as: Cong Y, Honda M, Fujishima K, Osugi J, Fujiu K. Perioperative management and surgical procedure for prolonged air leak: a clinical practice review. Shanghai Chest 2023;7:7.


